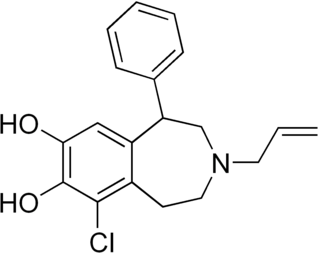
SKF-82,958 is a synthetic compound of the benzazepine class that acts as a D1/D5 receptor full agonist. SKF-82,958 and similar D1-like-selective full agonists like SKF-81,297 and 6-Br-APB produce characteristic anorectic effects, hyperactivity and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine. SKF-82,958 was also subsequently found to act as an agonist of ERα with negligible activity at ERβ, making it a subtype-selective estrogen.

Eletriptan, sold under the brand name Relpax and used in the form of eletriptan hydrobromide, is a second-generation triptan medication intended for treatment of migraine headaches. It is used as an abortive medication, blocking a migraine attack which is already in progress. Eletriptan is marketed and manufactured by Pfizer Inc.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

Zolazepam (Flupyrazapon) is a pyrazolodiazepinone derivative structurally related to the benzodiazepine drugs, which is used as an anaesthetic for a wide range of animals in veterinary medicine. Zolazepam is usually administered in combination with other drugs such as the NMDA antagonist tiletamine or the α2 adrenergic receptor agonist xylazine, depending on what purpose it is being used for. It is 5-10 times the potency of diazepam.

SL651498 is an anxiolytic and anticonvulsant drug used in scientific research, with a chemical structure most closely related to β-carboline derivatives such as abecarnil and gedocarnil. It has similar effects to benzodiazepine drugs, but is structurally distinct and so is classed as a nonbenzodiazepine anxiolytic.

EMD-386088 is an indole derivative which is used in scientific research. It acts as a potent 5-HT6 receptor partial agonist, with a Ki of 1 nM, a significantly higher affinity than older 5-HT6 agonists such as EMDT, although it possesses moderate affinity for the 5-HT3 receptor as well. Subsequent research has determined that EMD-386088 is also a dopamine reuptake inhibitor and that this action is involved in the antidepressant-like effects of the drug in rodents.

AL-34662 is an indazole derivative drug that is being developed for the treatment of glaucoma. It acts as a selective 5-HT2A receptor agonist, the same target as that of psychedelic drugs like psilocin, but unlike these drugs, AL-34662 was designed specifically as a peripherally selective drug, which does not cross the blood–brain barrier. This means that AL-34662 can exploit a useful side effect of the hallucinogenic 5-HT2A agonists, namely reduction in intra-ocular pressure and hence relief from the symptoms of glaucoma, but without causing the hallucinogenic effects that make centrally active 5-HT2A agonists unsuitable for clinical use. In animal studies, AL-34662 has been shown to be potent and effective in the treatment of symptoms of glaucoma, with minimal side effects.

PNU-22394 is a drug which acts as an agonist at serotonin 5-HT2 receptors, with strongest binding affinity for 5-HT2A and 5-HT2C and slightly weaker at 5-HT2B, although it is only a full agonist at 5-HT2C, but partial agonist at 5-HT2A and 5-HT2B. It has anorectic effects in both animal studies and human trials, along with "Pro-Cognitive Properties", although it has never been developed for medical use.

SKF-83,959, a synthetic benzazepine derivative used in scientific research, acts as an agonist at the D1–D2 dopamine receptor heteromer. It behaves as a full agonist at the D1 protomer and a high-affinity partial agonist at the D2 protomer. It was further shown to act as an allosteric modulator of the sigma-1 receptor. SKF-83,959 additionally inhibits sodium channels as well as delayed rectifier potassium channels. SKF-83,959 is a racemate that consists of the R-(+)- and S-(−)-enantiomers MCL-202 and MCL-201, respectively.

6-Br-APB is a synthetic compound that acts as a selective D1 agonist, with the (R)-enantiomer being a potent full agonist, while the (S) enantiomer retains its D1 selectivity but is a weak partial agonist. (R)-6-Br-APB and similar D1-selective full agonists like SKF-81,297 and SKF-82,958 produce characteristic anorectic effects, stereotyped behaviour and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine.

A-834,735 is a drug developed by Abbott Laboratories that acts as a potent cannabinoid receptor full agonist at both the CB1 and CB2 receptors, with a Ki of 12 nM at CB1 and 0.21 nM at CB2. Replacing the aromatic 3-benzoyl or 3-naphthoyl group found in most indole derived cannabinoids with the 3-tetramethylcyclopropylmethanone group of A-834,735 and related compounds imparts significant selectivity for CB2, with most compounds from this group found to be highly selective CB2 agonists with little affinity for CB1. However, low nanomolar CB1 binding affinity is retained with certain heterocyclic 1-position substituents such as (N-methylpiperidin-2-yl)methyl (cf. AM-1220, AM-1248), or the (tetrahydropyran-4-yl)methyl substituent of A-834,735, resulting in compounds that still show significant affinity and efficacy at both receptors despite being CB2 selective overall.

2,3,4,5-Tetrahydro-1,5-methano-1H-3-benzazepine is a drug originally researched as a potential opioid analgesic, but was found to be inactive in this assay, and relatively toxic to mice. Subsequently it was found to possess activity as an agonist at nicotinic acetylcholine receptors during the course of work that ultimately led to the discovery of the anti-smoking drug varenicline.
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb, that acts as a reasonably selective agonist of peripheral cannabinoid receptors. It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2, which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.

APICA is an indole based drug that acts as a potent agonist for the cannabinoid receptors.
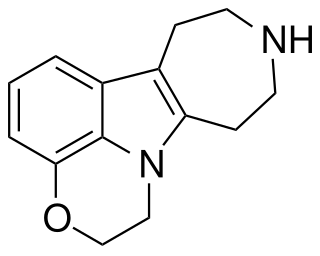
PHA-57378 is a drug which acts as an agonist at serotonin 5-HT2 receptors, having a binding affinity of 4.1 nM at the 5-HT2A subtype and 4.3 nM at 5-HT2C. It has anxiolytic effects in animal studies.
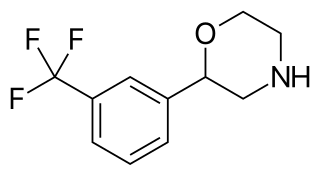
Flumexadol (INN) is a drug described and researched as a non-opioid analgesic which was never marketed. It has been found to act as an agonist of the serotonin 5-HT1A and 5-HT2C receptors and, to a much lesser extent, of the 5-HT2A receptor. According to Nilsson (2006) in a paper on 5-HT2C receptor agonists as potential anorectics, "The (+)-enantiomer of this compound showed [...] affinity for the 5-HT2C receptor (Ki) 25 nM) [...] and was 40-fold selective over the 5-HT2A receptor in receptor binding studies. Curiously, the racemic version [...], also known as 1841 CERM, was originally reported to possess analgesic properties while no association with 5-HT2C receptor activity was mentioned." It is implied that flumexadol might be employable as an anorectic in addition to analgesic. Though flumexadol itself has never been approved for medical use, oxaflozane is a prodrug of the compound that was formerly used clinically in France as an antidepressant and anxiolytic agent.

ADB-FUBHQUCA is a synthetic cannabinoid receptor agonist that has been sold as a designer drug, first reported in 2022. It is related to the previously reported compound ADB-FUBICA but with the central indole ring system expanded to a 1,4-dihydroquinoline structure. This breaks the aromaticity of the ring system, and ADB-FUBHQUCA is relatively low in potency compared to related compounds where the aromatic core is retained.

DM-506 (Ibogaminalog) is a drug first invented in the 1960s, which acts as both a partial agonist at the 5-HT2A receptor, and a negative allosteric modulator at the α7 and α9α10 nicotinic acetylcholine receptors. It can be regarded as a structurally simplified derivative of ibogaine and has been researched both for anti-addictive effects and for the treatment of neuropathic pain.



















