
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

The serotonin transporter also known as the sodium-dependent serotonin transporter and solute carrier family 6 member 4 is a protein that in humans is encoded by the SLC6A4 gene. SERT is a type of monoamine transporter protein that transports the neurotransmitter serotonin from the synaptic cleft back to the presynaptic neuron, in a process known as serotonin reuptake.

Ketanserin (INN, USAN, BAN) (brand name Sufrexal; former developmental code name R41468) is a drug used clinically as an antihypertensive agent and in scientific research to study the serotonergic system; specifically, the 5-HT2 receptor family. It was discovered at Janssen Pharmaceutica in 1980. It is not available in the United States.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

DASB, also known as 3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile, is a compound that binds to the serotonin transporter. Labeled with carbon-11 — a radioactive isotope — it has been used as a radioligand in neuroimaging with positron emission tomography (PET) since around year 2000. In this context it is regarded as one of the superior radioligands for PET study of the serotonin transporter in the brain, since it has high selectivity for the serotonin transporter.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Nisoxetine, originally synthesized in the Lilly research laboratories during the early 1970s, is a potent and selective inhibitor for the reuptake of norepinephrine (noradrenaline) into synapses. It currently has no clinical applications in humans, although it was originally researched as an antidepressant. Nisoxetine is now widely used in scientific research as a standard selective norepinephrine reuptake inhibitor. It has been used to research obesity and energy balance, and exerts some local analgesia effects.
Jeffrey H. Meyer is a scientist and professor working with mood and anxiety disorders using neuroimaging at the Department of Psychiatry, University of Toronto. He is currently the head of the Neurochemical Imaging Program in Mood and Anxiety Disorders in the Brain Health Imaging Centre at the Campbell Family Mental Health Research Institute and is working as a Senior Scientist in the General and Health Systems Psychiatry Division at the Centre for Addiction and Mental Health. He has also been awarded with the Tier 1 Canada Research Chair in the Neurochemistry of Major Depression.

Setoperone is a compound that is a ligand to the 5-HT2A receptor. It can be radiolabeled with the radioisotope fluorine-18 and used as a radioligand with positron emission tomography (PET). Several research studies have used the radiolabeled setoperone in neuroimaging for the studying neuropsychiatric disorders, such as depression or schizophrenia.

5-I-R91150 is a compound that acts as a potent and selective antagonist of 5-HT2A receptors. Its main application is as its iodine-123 radiolabeled form, in which it can be used in SPECT scanning in human neuroimaging studies, to examine the distribution of the 5-HT2A receptor subtype in the brain, e.g. with respect to sex and age and in adults with Asperger syndrome or Alzheimer's disease.

Volinanserin (INN) is a highly selective 5-HT2A receptor antagonist that is frequently used in scientific research to investigate the function of the 5-HT2A receptor. It was also tested in clinical trials as a potential antipsychotic, antidepressant, and treatment for insomnia but was never marketed.

25B-NBOMe is a derivative of the phenethylamine psychedelic 2C-B, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent full agonist for the 5HT2A receptor. Duration of effects lasts about 3–10 hours, although the parent compound is rapidly cleared from the blood when used in the radiolabeled form in tracer doses. Recently, Custodio et al. (2019) evaluated the potential involvement of dysregulated dopaminergic system, neuroadaptation, and brain wave changes which may contribute to the rewarding and reinforcing properties of 25B-NBOMe in rodents.
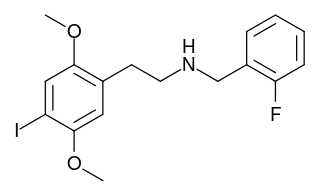
25I-NBF is a derivative of the phenethylamine hallucinogen 2C-I, which acts as a highly potent partial agonist for the human 5-HT2A receptor, with bias towards the β-arrestin 2 coupled signalling pathway. It has been studied in its 11C radiolabelled form as a potential ligand for mapping the distribution of 5-HT2A receptors in the brain, using positron emission tomography (PET).

25TFM-NBOMe is a derivative of the phenethylamine hallucinogen 2C-TFM, discovered in 2004 by Ralf Heim at the Free University of Berlin. It acts as a potent partial agonist for the 5-HT2A receptor, though its relative potency is disputed, with some studies finding it to be of lower potency than 25I-NBOMe, while others show it to be of similar or higher potency, possibly because of differences in the assay used. 2C-TFM-NB2OMe can be taken to produce psychedelic effects similar to 2C-I-NB2OMe and 2C-D-NB2OMe.

Brain positron emission tomography is a form of positron emission tomography (PET) that is used to measure brain metabolism and the distribution of exogenous radiolabeled chemical agents throughout the brain. PET measures emissions from radioactively labeled metabolically active chemicals that have been injected into the bloodstream. The emission data from brain PET are computer-processed to produce multi-dimensional images of the distribution of the chemicals throughout the brain.

Mefway is a serotonin 5-HT1A receptor antagonist used in medical research, usually in the form of mefway (18F) as a positron emission tomography (PET) radiotracer.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.
Nifene is a high affinity, selective nicotinic α4β2* receptor partial agonist used in medical research for nicotinic acetylcholine receptors, usually in the form of nifene (18F) as a positron emission tomography (PET) radiotracer.

Desmethoxyfallypride is a moderate affinity dopamine D2 receptor/D3 receptor antagonist used in medical research, usually in the form of the radiopharmaceutical [F-18]-desmethoxyfallypride (DMFP(18F)) which has been used in human studies as a positron emission tomography (PET) radiotracer.

The 25-NB (25x-NBx) series, sometimes alternatively referred to as the NBOMe compounds, is a family of serotonergic psychedelics. They are substituted phenethylamines and were derived from the 2C family. They act as selective agonists of the serotonin 5-HT2A receptor. The 25-NB family is unique relative to other classes of psychedelics in that they are, generally speaking, extremely potent and relatively selective for the 5-HT2A receptor. Use of NBOMe series drugs has caused many deaths and hospitalisations since the drugs popularisation in the 2010s. This is primarily due to their high potency, unpredictable pharmacokinetics, and sellers passing off the compounds in the series as LSD.





















