
Irritable bowel syndrome (IBS) is a "disorder of gut-brain interaction" characterized by a group of symptoms that commonly include abdominal pain, abdominal bloating and changes in the consistency of bowel movements. These symptoms may occur over a long time, sometimes for years. IBS can negatively affect quality of life and may result in missed school or work or reduced productivity at work. Disorders such as anxiety, major depression, and chronic fatigue syndrome are common among people with IBS.

Ondansetron, sold under the brand name Zofran among others, is a medication used to prevent nausea and vomiting caused by cancer chemotherapy, radiation therapy, or surgery. It is also effective for treating gastroenteritis. It can be given orally, intramuscularly, or intravenously.

Alosetron, sold under the brand name Lotronex among others, is a 5-HT3 antagonist used for the management of severe diarrhea-predominant irritable bowel syndrome (IBS) in females only.

Tegaserod is a 5-HT4 agonist manufactured by Novartis and sold under the names Zelnorm and Zelmac for the management of irritable bowel syndrome and constipation. Approved by the FDA in 2002, it was subsequently removed from the market in 2007 due to FDA concerns about possible adverse cardiovascular effects. Before then, it was the only drug approved by the United States Food and Drug Administration to help relieve the abdominal discomfort, bloating, and constipation associated with irritable bowel syndrome. Its use was also approved to treat chronic idiopathic constipation.

Small intestinal bacterial overgrowth (SIBO), also termed bacterial overgrowth, or small bowel bacterial overgrowth syndrome (SBBOS), is a disorder of excessive bacterial growth in the small intestine. Unlike the colon, which is rich with bacteria, the small bowel usually has fewer than 100,000 organisms per millilitre. Patients with bacterial overgrowth typically develop symptoms which may include nausea, bloating, vomiting, diarrhea, malnutrition, weight loss and malabsorption, which is caused by a number of mechanisms.
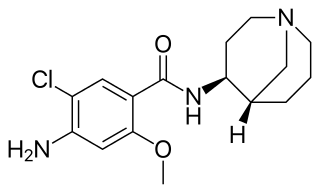
Renzapride is a prokinetic agent and antiemetic which acts as a full 5-HT4 agonist and partial 5-HT3 antagonist. It also functions as a 5-HT2B antagonist and has some affinity for the 5-HT2A and 5-HT2C receptors.

Rifaximin, is a non-absorbable, broad spectrum antibiotic mainly used to treat travelers' diarrhea. It is based on the rifamycin antibiotics family. Since its approval in Italy in 1987, it has been licensed in over more than 30 countries for the treatment of a variety of gastrointestinal diseases like irritable bowel syndrome, and hepatic encephalopathy. It acts by inhibiting RNA synthesis in susceptible bacteria by binding to the RNA polymerase enzyme. This binding blocks translocation, which stops transcription. It is marketed under the brand name Xifaxan by Salix Pharmaceuticals.

Lubiprostone, sold under the brand name Amitiza among others, is a medication used in the management of chronic idiopathic constipation, predominantly irritable bowel syndrome-associated constipation in women and opioid-induced constipation. The drug is owned by Mallinckrodt and is marketed by Takeda Pharmaceutical Company.

The 5-HT3 antagonists, informally known as "setrons", are a class of drugs that act as receptor antagonists at the 5-HT3 receptor, a subtype of serotonin receptor found in terminals of the vagus nerve and in certain areas of the brain. With the notable exceptions of alosetron and cilansetron, which are used in the treatment of irritable bowel syndrome, all 5-HT3 antagonists are antiemetics, used in the prevention and treatment of nausea and vomiting. They are particularly effective in controlling the nausea and vomiting produced by cancer chemotherapy and are considered the gold standard for this purpose.

Polycarbophil calcium (INN) is a drug used as a stool stabilizer. Chemically, it is a synthetic polymer of polyacrylic acid cross-linked with divinyl glycol, with calcium as a counter-ion.
A serotonin antagonist, or serotonin receptor antagonist, is a drug used to inhibit the action of serotonin and serotonergic drugs at serotonin (5-HT) receptors.

Ramosetron (INN) is a serotonin 5-HT3 receptor antagonist for the treatment of nausea and vomiting. Ramosetron is also indicated for a treatment of "diarrhea-predominant irritable bowel syndrome in male and women". In India it is marketed under the brand name of Ibset.

5-hydroxytryptamine receptor 3E is a protein that in humans is encoded by the HTR3E gene. The protein encoded by this gene is a subunit of the 5-HT3 receptor.

Mepiprazole is an anxiolytic drug of the phenylpiperazine group with additional antidepressant properties that is marketed in Spain. It acts as a 5-HT2A and α1-adrenergic receptor antagonist and inhibits the reuptake and induces the release of serotonin, dopamine, and norepinephrine to varying extents, and has been described as a serotonin antagonist and reuptake inhibitor (SARI). Controlled clinical trials of mepiprazole in patients with irritable bowel syndrome (IBS) were also carried out and suggested some benefits of the drug in relieving symptoms of IBS in some patients. Similarly to other phenylpiperazines like trazodone, nefazodone, and etoperidone, mepiprazole produces mCPP as an active metabolite.
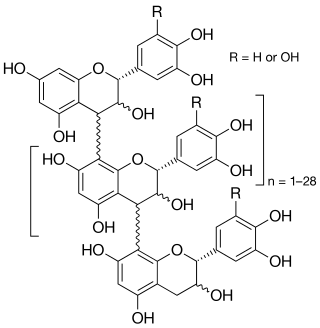
Crofelemer is an antidiarrheal indicated for the symptomatic relief of non-infectious diarrhea in adult patients with HIV/AIDS on antiretroviral therapy. Other possible uses include diarrhea in children, acute infectious diarrhea, and diarrhea in patients with irritable bowel syndrome. It is a purified oligomeric proanthocyanidin from "dragon's blood", the sap of the South American tree Croton lechleri.
Serum-derived bovine immunoglobulin/protein isolate (SBI) is a medical food product derived from bovine serum obtained from adult cows in the United States. It is sold under the name EnteraGam.

Eluxadoline, sold under the brand names Viberzi and Truberzi, is a medication taken by mouth for the treatment of diarrhea and abdominal pain in individuals with diarrhea-predominant irritable bowel syndrome (IBS-D). It was approved for use in the United States in 2015. The drug originated from Janssen Pharmaceutica and was developed by Actavis.
Peripherally selective drugs have their primary mechanism of action outside of the central nervous system (CNS), usually because they are excluded from the CNS by the blood–brain barrier. By being excluded from the CNS, drugs may act on the rest of the body without producing side-effects related to their effects on the brain or spinal cord. For example, most opioids cause sedation when given at a sufficiently high dose, but peripherally selective opioids can act on the rest of the body without entering the brain and are less likely to cause sedation. These peripherally selective opioids can be used as antidiarrheals, for instance loperamide (Imodium).
Plecanatide, sold under the brand name Trulance, is a medication for the treatment of chronic idiopathic constipation (CIC) and irritable bowel syndrome with constipation. It is being launched in India under the brand name "Plecasoft". Plecanatide is an agonist of guanylate cyclase-C. Plecanatide increases intestinal transit and fluid through a buildup of cGMP.

Olorinab (APD371) is a drug being developed by Arena Pharmaceuticals for the treatment of gastrointestinal pain associated with Crohn's disease and irritable bowel syndrome. It acts as a potent and selective cannabinoid CB2 receptor agonist and is claimed to be orally active and peripherally selective. Initial Phase IIa exploratory clinical trials have been successful in patients with quiescent Crohn's disease. Arena initiated the Phase IIb Captivate trial in late July 2019 in patients with irritable bowel syndrome related pain, in constipation and diarrhea predominant sub-types. The Phase IIb trial is expected to enroll 240 participants between the ages of 18 and 70.Three doses of 10 mg, 25 mg, and 50 mg are being tested against Placebo in a 3:4 prescription ratio with a Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) masking layout.















