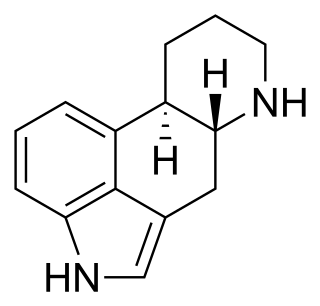
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.
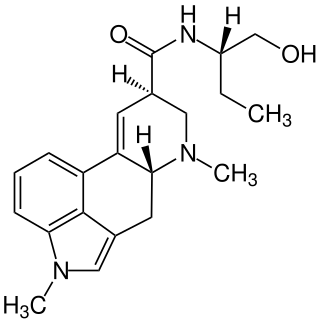
Methysergide, sold under the brand names Deseril and Sansert, is a monoaminergic medication of the ergoline and lysergamide groups which is used in the prophylaxis and treatment of migraine and cluster headaches. It has been withdrawn from the market in the United States and Canada due to adverse effects. It is taken by mouth.
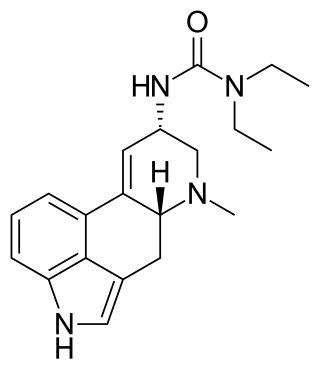
Lisuride, sold under the brand name Dopergin among others, is a monoaminergic medication of the ergoline class which is used in the treatment of Parkinson's disease, migraine, and high prolactin levels. It is taken by mouth.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2B receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.

4-Substituted-2,5-dimethoxyamphetamines (DOx) is a chemical class of substituted amphetamine derivatives featuring methoxy groups at the 2- and 5- positions of the phenyl ring, and a substituent such as alkyl or halogen at the 4- position of the phenyl ring. Most compounds of this class are potent and long-lasting psychedelic drugs, and act as highly selective 5-HT2A, 5-HT2B, and 5-HT2C receptor partial agonists. A few bulkier derivatives such as DOAM have similarly high binding affinity for 5-HT2 receptors but instead act as antagonists, and so do not produce psychedelic effects though they retain amphetamine-like stimulant effects.

Adatanserin is a mixed 5-HT1A receptor partial agonist and 5-HT2A and 5-HT2C receptor antagonist. It was under development by Wyeth as an antidepressant but was ultimately not pursued.

RH-34 is a compound which acts as a potent and selective partial agonist for the 5-HT2A serotonin receptor subtype. It was derived by structural modification of the selective 5-HT2A antagonist ketanserin, with the 4-(p-fluorobenzoyl)piperidine moiety replaced by the N-(2-methoxybenzyl) pharmacophore found in such potent 5-HT2A agonists as NBOMe-2C-B and NBOMe-2C-I. This alteration was found to retain 5-HT2A affinity and selectivity, but reversed activity from an antagonist to a moderate efficacy partial agonist.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

The 25-NB (25x-NBx) series, sometimes alternatively referred to as the NBOMe compounds, is a family of serotonergic psychedelics. They are substituted phenethylamines and were derived from the 2C family. They act as selective agonists of the serotonin 5-HT2A receptor. The 25-NB family is unique relative to other classes of psychedelics in that they are, generally speaking, extremely potent and relatively selective for the 5-HT2A receptor. Use of NBOMe series drugs has caused many deaths and hospitalisations since the drugs popularisation in the 2010s. This is primarily due to their high potency, unpredictable pharmacokinetics, and sellers passing off the compounds in the series as LSD.

WAY-163,909 is a drug which acts as a potent and reasonably selective agonist for the serotonin 5-HT2C receptor. It has antipsychotic-like effects in animal models, and has been used to study the role of the 5-HT2C receptor subtype in the action of addictive drugs such as nicotine and methamphetamine.

AAZ-A-154 is a novel isotryptamine derivative which acts as a 5-HT2A receptor agonist discovered and synthesized by the lab of Professor David E. Olson at UCDavis. Animal studies suggest that it produces antidepressant effects without the psychedelic action typical of drugs from this class. In tests, AAZ-A-154 had antidepressant effects in mice without causing the head-twitch response linked to hallucinogenic effects. Due to the rapidly-induced and enduring neuroplasticity, AAZ-A-154 is a member of the class of compounds known as non-hallucinogenic psychoplastogens. This compound, as well as related compounds, are licensed by Delix Therapeutics and are being developed as potential medicines for neuropsychiatric disorders.

Z3517967757, or simply Z7757, is a piperidine derivative which acts as an agonist at the 5-HT2 family of serotonin receptors, first reported in 2024. It can also be viewed as a ring-restrained phenethylamine. It has strongest activity at the 5-HT2A receptor and lower affinity at the 5-HT2B and 5-HT2C receptors. However, it has been reported to have excellent selectivity for the 5-HT2A receptor, with no agonistic activity at the 5-HT2B and 5-HT2C receptors. The drug was developed using in silico modelling to dock a large library of compounds against a 5-HT2A receptor model generated by the artificial intelligence program AlphaFold, and then synthesised and tested in the laboratory to confirm activity. It has two stereogenic centers and four possible isomers, but has only been tested as a racemic mixture.
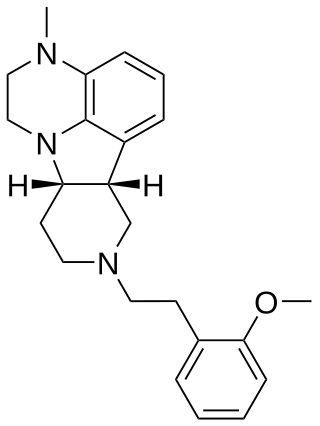
IHCH-7079 is a drug which acts as an agonist at the 5-HT2A serotonin receptor. It was derived by structural simplification of the 5-HT2A antagonist atypical antipsychotic drug lumateperone along with several related compounds such as IHCH-7086, which was found to be a non-hallucinogenic biased 5-HT2A agonist that was active in antidepressant assays but did not produce psychedelic-like responding in mice. The related structure IHCH-7113 was found to produce a positive head-twitch response in mice, suggesting likely hallucinogenic activity in humans.

IHCH-7086 is a drug which acts as an agonist at the 5-HT2A serotonin receptor. It was derived by structural simplification of the 5-HT2A antagonist atypical antipsychotic drug lumateperone along with several related compounds such as IHCH-7079, which was found to be a non-hallucinogenic biased 5-HT2A agonist that was active in antidepressant assays but did not produce psychedelic-like responding in mice. The related structure IHCH-7113 was found to produce a positive head-twitch response in mice, suggesting likely hallucinogenic activity in humans.

ITI-333 is a drug which has a mixed mechanism of action, acting as an antagonist at the 5-HT2A, D1 and α1A receptors, and also as a partial agonist at the μ-opioid receptor. In animal studies it blocked the head-twitch response produced by DOI and also reduced responses to morphine, while also reducing the symptoms produced by naloxone-precipitated withdrawal in opioid habituated mice. It has been developed for potential uses in treatment of opioid withdrawal and opioid use disorder.
A trip killer, or hallucinogen antidote, is a drug that aborts or reduces the effects of a hallucinogenic drug experience. As there are different types of hallucinogens that work in different ways, there are different types of trip killers. They can completely block or reduce the effects of hallucinogens or they can simply provide anxiety relief and sedation. Examples of trip killers, in the case of serotonergic psychedelics, include serotonin receptor antagonists, like antipsychotics and certain antidepressants, and benzodiazepines. Trip killers are sometimes used by recreational psychedelic users as a form of harm reduction to manage so-called bad trips, for instance difficult experiences with prominent anxiety. They can also be used clinically to manage effects of hallucinogens, like anxiety and psychomotor agitation, for instance in the emergency department.
ITI-1549 is a putatively non-hallucinogenic serotonin 5-HT2A receptor agonist which is under development for the treatment of mood disorders and other psychiatric disorders. In addition to acting at the serotonin 5-HT2A receptor, it is also an antagonist of the serotonin 5-HT2B receptor and an agonist of the serotonin 5-HT2C receptor. The drug's route of administration has not been specified.

















