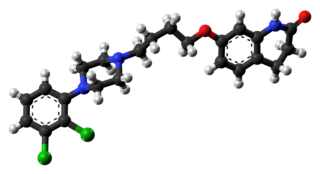
Aripiprazole, sold under the brand names Abilify and Aristada, among others, is an atypical antipsychotic. It is primarily used in the treatment of schizophrenia and bipolar disorder; other uses include as an add-on treatment in major depressive disorder and obsessive–compulsive disorder (OCD), tic disorders, and irritability associated with autism. Aripiprazole is taken by mouth or via injection into a muscle. A Cochrane review found low-quality evidence of effectiveness in treating schizophrenia.
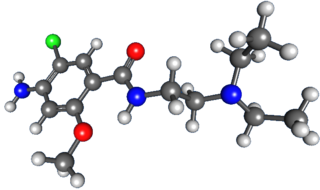
Metoclopramide is a medication used for stomach and esophageal problems. It is commonly used to treat and prevent nausea and vomiting, to help with emptying of the stomach in people with delayed stomach emptying, and to help with gastroesophageal reflux disease. It is also used to treat migraine headaches.
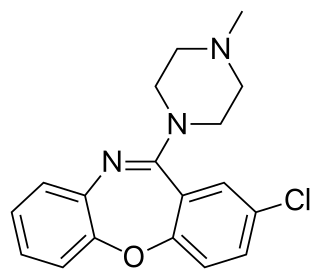
Loxapine, sold under the brand names Loxitane and Adasuve among others, is a tricyclic antipsychotic medication used primarily in the treatment of schizophrenia. The medicine is a member of the dibenzoxazepine class and structurally very similar to clozapine. Several researchers have argued that loxapine, initially classified as a typical antipsychotic, behaves as an atypical antipsychotic.

Granisetron is a serotonin 5-HT3 receptor antagonist used as an antiemetic to treat nausea and vomiting following chemotherapy and radiotherapy. Its main effect is to reduce the activity of the vagus nerve, which is a nerve that activates the vomiting center in the medulla oblongata. It does not have much effect on vomiting due to motion sickness. This drug does not have any effect on dopamine receptors or muscarinic receptors.
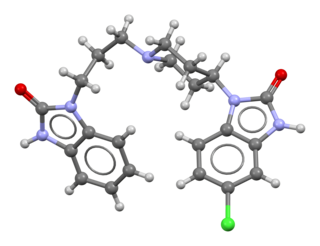
Domperidone, sold under the brand name Motilium among others, is a dopamine antagonist medication which is used to treat nausea and vomiting and certain gastrointestinal problems like gastroparesis. It raises the level of prolactin in the human body and is used off label to induce and promote breast milk production. It may be taken by mouth or rectally.

Tegaserod is a 5-HT4 agonist manufactured by Novartis and sold under the names Zelnorm and Zelmac for the management of irritable bowel syndrome and constipation. Approved by the FDA in 2002, it was subsequently removed from the market in 2007 due to FDA concerns about possible adverse cardiovascular effects. Before then, it was the only drug approved by the United States Food and Drug Administration to help relieve the abdominal discomfort, bloating, and constipation associated with irritable bowel syndrome. Its use was also approved to treat chronic idiopathic constipation.
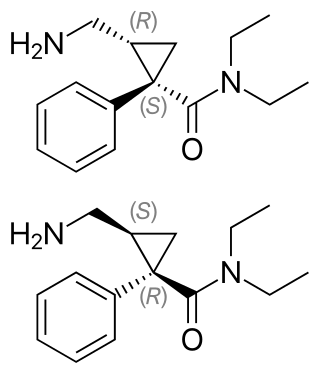
Milnacipran is a serotonin–norepinephrine reuptake inhibitor (SNRI) used in the clinical treatment of fibromyalgia. It is not approved for the clinical treatment of major depressive disorder in the US, but it is in other countries.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.
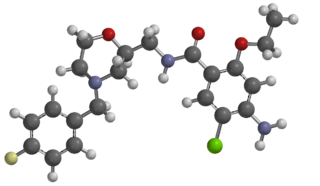
Mosapride is a gastroprokinetic agent that acts as a selective 5HT4 agonist. The major active metabolite of mosapride, known as M1, additionally acts as a 5HT3 antagonist, which accelerates gastric emptying throughout the whole of the gastrointestinal tract in humans, and is used for the treatment of gastritis, gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome. It is recommended to be taken on an empty stomach (i.e. at least one hour before food or two hours after food).

5-Hydroxytryptamine receptor 4 is a protein that in humans is encoded by the HTR4 gene.

Itopride (INN; brand name Ganaton) is a prokinetic benzamide derivative. These drugs inhibit dopamine and acetylcholine esterase enzyme and have a gastrokinetic effect. Itopride is indicated for the treatment of functional dyspepsia and other gastrointestinal conditions. It is a combined D2 receptor antagonist and acetylcholinesterase inhibitor. Itopride is the dimethoxy analog of trimethobenzamide.

Prucalopride, sold under brand names Resolor and Motegrity among others, is a medication acting as a selective, high affinity 5-HT4 receptor agonist which targets the impaired motility associated with chronic constipation, thus normalizing bowel movements. Prucalopride was approved for medical use in the European Union in 2009, in Canada in 2011, in Israel in 2014, and in the United States in December 2018. The drug has also been tested for the treatment of chronic intestinal pseudo-obstruction.

Levosulpiride, sold under the brand name SULPEPTA , is a potent prokinetic agent of the benzamide class. It is a selective antagonist of the dopamine D2 receptors and 5-HT4 agonist on both central and peripheral nervous systems. Levosulpiride is claimed to have mood elevating properties.

Cinitapride (trade names Cintapro, Pemix) is a gastroprokinetic agent and antiemetic agent of the benzamide class which is marketed in India, Mexico, Pakistan and Spain. It acts as an agonist of the 5-HT1 and 5-HT4 receptors and as an antagonist of the 5-HT2 receptors.

Dazopride (AHR-5531) is an antiemetic and gastroprokinetic agent of the benzamide class which was never marketed. It acts as a 5-HT3 receptor antagonist and 5-HT4 receptor agonist. In addition to its gastrointestinal effects, dazopride facilitates learning and memory in mice.
A prokinetic agent is a type of small peptide drug which enhances gastrointestinal motility by increasing the frequency or strength of contractions, but without disrupting their rhythm. They are used to treat certain gastrointestinal symptoms, including abdominal discomfort, bloating, constipation, heart burn, nausea, and vomiting; and certain gastrointestinal disorders, including irritable bowel syndrome, gastritis, gastroparesis, and functional dyspepsia.
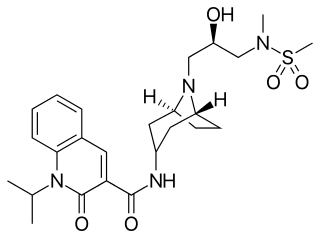
Velusetrag (INN, USAN; previously known as TD-5108) is an experimental drug candidate for the treatment of gastric neuromuscular disorders including gastroparesis, and lower gastrointestinal motility disorders including chronic idiopathic constipation and irritable bowel syndrome. It is a potent, selective, high efficacy 5-HT4 receptor serotonin agonist being developed by Theravance Biopharma and Alfa Wassermann. Velusetrag demonstrates less selectivity for other serotonin receptors, such as 5-HT2 and 5-HT3, to earlier generation 5-HT agonists like cisapride and tegaserod.
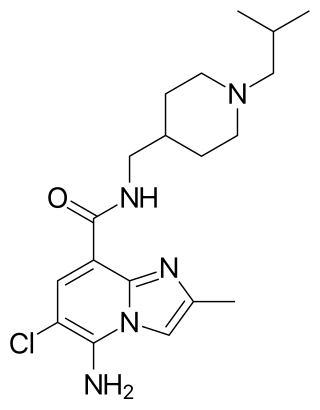
CJ-033466 is a drug which acts as a potent and selective 5-HT4 serotonin receptor partial agonist. In animal tests it stimulated gastrointestinal motility with 30 times the potency of cisapride, and with lower affinity for the hERG channel.
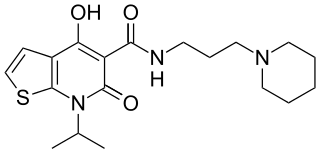
PRX-03140 is an orally-bioavailable selective partial agonist to the 5-Hydroxytryptamine receptor 4 (5-HT4) and a ligand for the Sigma-1 and Sigma-2 receptors. PRX-03140 is a partial agonist (18% relative to 5-HT) of the 5-HT4 receptor that was developed by EPIX Pharmaceuticals for Alzheimer's disease. PRX-03140 is a highly selective and potent (Ki = 22-37 nM) 5-HT4R agonist in radioligand binding assays with more than 100-fold difference in affinities compared with all other 5-HT receptors tested. PRX-03140 behaves as a partial agonist in cell lines expressing either the human 5-HT4aR, 5-HT4bR or 5-HT4eR isoforms, stimulating cAMP production to 30%-60% compared to 5-HT. PRX-03140 also demonstrates binding to both the Sigma-1 and Sigma-2 receptors (Ki = 79-100 nM and 99-160 nM, respectively) in radioligand binding assays, but demonstrates no significant affinity for more than 50 other receptors tested including GPCRs, ion channels and receptor tyrosine kinases. PRX-03140 demonstrates high CNS penetration without inducing significant distal gastrointestinal motility observed with gastrointestinally active 5-HT4 agonists (e.g. cisapride, tegaserod).
Peripherally acting μ-opioid receptor antagonists (PAMORAs) are a class of chemical compounds that are used to reverse adverse effects caused by opioids interacting with receptors outside the central nervous system (CNS), mainly those located in the gastrointestinal tract. PAMORAs are designed to specifically inhibit certain opioid receptors in the gastrointestinal tract and with limited ability to cross the blood–brain barrier. Therefore, PAMORAs do not affect the analgesic effects of opioids within the central nervous system.



















