Hypertrophic cardiomyopathy is a condition in which muscle tissues of the heart become thickened without an obvious cause. The parts of the heart most commonly affected are the interventricular septum and the ventricles. This results in the heart being less able to pump blood effectively and also may cause electrical conduction problems. Specifically, within the bundle branches that conduct impulses through the interventricular septum and into the Purkinje fibers, as these are responsible for the depolarization of contractile cells of both ventricles.
An inotrope or inotropic is a drug or any substance that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.

Diltiazem, sold under the brand name Cardizem among others, is a nondihydropyridine calcium channel blocker medication used to treat high blood pressure, angina, and certain heart arrhythmias. It may also be used in hyperthyroidism if beta blockers cannot be used. It is taken by mouth or injection into a vein. When given by injection, effects typically begin within a few minutes and last a few hours.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.
Pulsus paradoxus, also paradoxic pulse or paradoxical pulse, is an abnormally large decrease in stroke volume, systolic blood pressure and pulse wave amplitude during inspiration. Pulsus paradoxus is not related to pulse rate or heart rate, and it is not a paradoxical rise in systolic pressure. Normally, blood pressure drops less precipitously than 10 mmHg during inhalation. Pulsus paradoxus is a sign that is indicative of several conditions, most commonly pericardial effusion.
Tachycardia-induced cardiomyopathy (TIC) is a disease where prolonged tachycardia or arrhythmia causes an impairment of the myocardium, which can result in heart failure. People with TIC may have symptoms associated with heart failure and/or symptoms related to the tachycardia or arrhythmia. Though atrial fibrillation is the most common cause of TIC, several tachycardias and arrhythmias have been associated with the disease.

Restrictive cardiomyopathy (RCM) is a form of cardiomyopathy in which the walls of the heart are rigid. Thus the heart is restricted from stretching and filling with blood properly. It is the least common of the three original subtypes of cardiomyopathy: hypertrophic, dilated, and restrictive.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.

Amrinone, also known as inamrinone, and sold as Inocor, is a pyridine phosphodiesterase 3 inhibitor. It is a drug that may improve the prognosis in patients with congestive heart failure. Amrinone has been shown to increase the contractions initiated in the heart by high-gain calcium induced calcium release (CICR). The positive inotropic effect of amrinone is mediated by the selective enhancement of high-gain CICR, which contributes to the contraction of myocytes by phosphorylation through cAMP dependent protein kinase A (PKA) and Ca2+ calmodulin kinase pathways.

Prajmaline (Neo-gilurythmal) is a class Ia antiarrhythmic agent which has been available since the 1970s. Class Ia drugs increase the time one action potential lasts in the heart. Prajmaline is a semi-synthetic propyl derivative of ajmaline, with a higher bioavailability than its predecessor. It acts to stop arrhythmias of the heart through a frequency-dependent block of cardiac sodium channels.

Takotsubo cardiomyopathy or takotsubo syndrome (TTS), also known as stress cardiomyopathy, is a type of non-ischemic cardiomyopathy in which there is a sudden temporary weakening of the muscular portion of the heart. It usually appears after a significant stressor, either physical or emotional; when caused by the latter, the condition is sometimes called broken heart syndrome.
Septal myectomy is a cardiac surgery treatment for hypertrophic cardiomyopathy (HCM). The open-heart surgery entails removing a portion of the septum that is obstructing the flow of blood from the left ventricle to the aorta.
Alcohol septal ablation (ASA) is a minimally invasive heart procedure to treat hypertrophic cardiomyopathy (HCM).

Noncompaction cardiomyopathy (NCC) is a rare congenital disease of heart muscle that affects both children and adults. It results from abnormal prenatal development of heart muscle.
The following outline is provided as an overview of and topical guide to cardiology, the branch of medicine dealing with disorders of the human heart. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease and electrophysiology. Physicians who specialize in cardiology are called cardiologists.

Hypertrophic cardiomyopathy screening is an assessment and testing to detect hypertrophic cardiomyopathy (HCM).
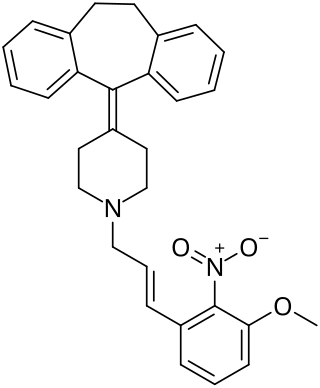
AH-1058 is a lipophilic antiarrhythmic calcium channel blocker synthesized by the Pharmaceutical Research Laboratories of Ajinomoto Co., Inc in Kawasaki, Japan. It is derived from cyproheptadine, a compound with known antiserotonic, antihistaminic and calcium channel blocking properties. The IUPAC name of AH-1058 is: 4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-[E-3-(3-methoxy-2-nitro) phenyl-2-propenyl]piperidine hydrochloride.

Heart failure with preserved ejection fraction (HFpEF) is a form of heart failure in which the ejection fraction – the percentage of the volume of blood ejected from the left ventricle with each heartbeat divided by the volume of blood when the left ventricle is maximally filled – is normal, defined as greater than 50%; this may be measured by echocardiography or cardiac catheterization. Approximately half of people with heart failure have preserved ejection fraction, while the other half have a reduction in ejection fraction, called heart failure with reduced ejection fraction (HFrEF).

Istaroxime is an investigational drug under development for treatment of acute decompensated heart failure
Tissue Doppler echocardiography (TDE) is a medical ultrasound technology, specifically a form of echocardiography that measures the velocity of the heart muscle (myocardium) through the phases of one or more heartbeats by the Doppler effect of the reflected ultrasound. The technique is the same as for flow Doppler echocardiography measuring flow velocities. Tissue signals, however, have higher amplitude and lower velocities, and the signals are extracted by using different filter and gain settings. The terms tissue Doppler imaging (TDI) and tissue velocity imaging (TVI) are usually synonymous with TDE because echocardiography is the main use of tissue Doppler.













