Cardioversion is a medical procedure by which an abnormally fast heart rate (tachycardia) or other cardiac arrhythmia is converted to a normal rhythm using electricity or drugs. Synchronized electrical cardioversion uses a therapeutic dose of electric current to the heart at a specific moment in the cardiac cycle, restoring the activity of the electrical conduction system of the heart. Pharmacologic cardioversion, also called chemical cardioversion, uses antiarrhythmia medication instead of an electrical shock.
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a group of pharmaceuticals that are used to suppress abnormally fast rhythms (tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular tachycardia.

Quinidine is a class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of antimalarial agent quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval. As of 2019, its IV formulation is no longer being manufactured for use in the United States.
An inotrope or inotropic is a drug or any substance that alters the force or energy of muscular contractions. Negatively inotropic agents weaken the force of muscular contractions. Positively inotropic agents increase the strength of muscular contraction.
Amiodarone is an antiarrhythmic medication used to treat and prevent a number of types of cardiac dysrhythmias. This includes ventricular tachycardia (VT), ventricular fibrillation (VF), and wide complex tachycardia, as well as atrial fibrillation and paroxysmal supraventricular tachycardia. Evidence in cardiac arrest, however, is poor. It can be given by mouth, intravenously, or intraosseously. When used by mouth, it can take a few weeks for effects to begin.

Procainamide (PCA) is a medication of the antiarrhythmic class used for the treatment of cardiac arrhythmias. It is a sodium channel blocker of cardiomyocytes; thus it is classified by the Vaughan Williams classification system as class Ia. In addition to blocking the INa current, it inhibits the IKr rectifier K+ current. Procainamide is also known to induce a voltage-dependent open channel block on the batrachotoxin (BTX)-activated sodium channels in cardiomyocytes.
Proarrhythmia is a new or more frequent occurrence of pre-existing arrhythmias, paradoxically precipitated by antiarrhythmic therapy, which means it is a side effect associated with the administration of some existing antiarrhythmic drugs, as well as drugs for other indications. In other words, it is a tendency of antiarrhythmic drugs to facilitate emergence of new arrhythmias.
Azimilide is a class ΙΙΙ antiarrhythmic drug. The agents from this heterogeneous group have an effect on the repolarization, they prolong the duration of the action potential and the refractory period. Also they slow down the spontaneous discharge frequency of automatic pacemakers by depressing the slope of diastolic depolarization. They shift the threshold towards zero or hyperpolarize the membrane potential. Although each agent has its own properties and will have thus a different function.

Disopyramide is an antiarrhythmic medication used in the treatment of ventricular tachycardia. It is a sodium channel blocker and is classified as a Class 1a anti-arrhythmic agent. Disopyramide has a negative inotropic effect on the ventricular myocardium, significantly decreasing the contractility. Disopyramide also has an anticholinergic effect on the heart which accounts for many adverse side effects. Disopyramide is available in both oral and intravenous forms, and has a low degree of toxicity.

Ibutilide is a Class III antiarrhythmic agent that is indicated for acute cardioconversion of atrial fibrillation and atrial flutter of a recent onset to sinus rhythm. It exerts its antiarrhythmic effect by induction of slow inward sodium current, which prolongs action potential and refractory period of myocardial cells. Because of its Class III antiarrhythmic activity, there should not be concomitant administration of Class Ia and Class III agents.

Prajmaline (Neo-gilurythmal) is a class Ia antiarrhythmic agent which has been available since the 1970s. Class Ia drugs increase the time one action potential lasts in the heart. Prajmaline is a semi-synthetic propyl derivative of ajmaline, with a higher bioavailability than its predecessor. It acts to stop arrhythmias of the heart through a frequency-dependent block of cardiac sodium channels.

Lorcainide is a Class 1c antiarrhythmic agent that is used to help restore normal heart rhythm and conduction in patients with premature ventricular contractions, ventricular tachycardiac and Wolff–Parkinson–White syndrome. Lorcainide was developed by Janssen Pharmaceutica (Belgium) in 1968 under the commercial name Remivox and is designated by code numbers R-15889 or Ro 13-1042/001. It has a half-life of 8.9 +- 2.3 hrs which may be prolonged to 66 hrs in people with cardiac disease.

Pilsicainide (INN) is an antiarrhythmic agent. It is marketed in Japan as サンリズム (Sunrythm). It was developed by Suntory Holdings Limited and first released in 1991. The JAN applies to the hydrochloride salt, pilsicainide hydrochloride.

Arrhythmias, also known as cardiac arrhythmias, heart arrhythmias, or dysrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a resting heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath, chest pain, or decreased level of consciousness. While most cases of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death.

BRL-32872 is an experimental drug candidate that provides a novel approach to the treatment of cardiac arrhythmia. Being a derivative of verapamil, it possesses the ability to inhibit Ca+2 membrane channels. Specific modifications in hydrogen bonding activity, nitrogen lone pair availability, and molecular flexibility allow BRL-32872 to inhibit K+ channels as well. As such, BRL-32872 is classified as both a class III (K+ blocking) and class IV (Ca+2 blocking) antiarrhythmic agent.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.

Budiodarone (ATI-2042) is an antiarrhythmic agent and chemical analog of amiodarone that is currently being studied in clinical trials. Amiodarone is considered the most effective antiarrhythmic drug available, but its adverse side effects, including hepatic, pulmonary and thyroid toxicity as well as multiple drug interactions, are discouraging its use. Budiodarone only differs in structure from amiodarone through the presence of a sec-butyl acetate side chain at position 2 of the benzofuran moiety. This side chain allows for budiodarone to have a shorter half-life in the body than amiodarone which allows it to have a faster onset of action and metabolism while still maintaining similar electrophysiological activity. The faster metabolism of budiodarone allows for fewer adverse side effects than amiodarone principally due to decreased levels of toxicity in the body.

AZD1305 is an experimental drug candidate that is under investigation for the management and reversal of cardiac arrhythmias, specifically atrial fibrillation and flutter. In vitro studies have shown that this combined-ion channel blocker inhibits rapidly the activating delayed-rectifier potassium current (IKr), L-type calcium current, and inward sodium current (INa).
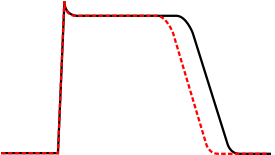
QT prolongation is a measure of delayed ventricular repolarisation, which means the heart muscle takes longer than normal to recharge between beats. It is an electrical disturbance which can be seen on an electrocardiogram (ECG). Excessive QT prolongation can trigger tachycardias such as torsades de pointes (TdP). QT prolongation is an established side effect of antiarrhythmics, but can also be caused by a wide range of non-cardiac medicines, including antibiotics, antidepressants, antihistamines, opioids, and complementary medicines. On an ECG, the QT interval represents the summation of action potentials in cardiac muscle cells, which can be caused by an increase in inward current through sodium or calcium channels, or a decrease in outward current through potassium channels. By binding to and inhibiting the “rapid” delayed rectifier potassium current protein, certain drugs are able to decrease the outward flow of potassium ions and extend the length of phase 3 myocardial repolarization, resulting in QT prolongation.