
5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Desmetramadol, also known as O-desmethyltramadol (O-DSMT), is an opioid analgesic and the main active metabolite of tramadol. Tramadol is demethylated by the liver enzyme CYP2D6 to desmetramadol in the same way as codeine, and so similarly to the variation in effects seen with codeine, individuals who have a less active form of CYP2D6 will tend to have reduced analgesic effects from tramadol. Because desmetramadol itself does not need to be metabolized to induce an analgesic effect, it can be used in individuals with low CYP2D6 activity unlike tramadol.

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80-100x selective for D3 over D2, and lacks any partial agonist activity.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Glutamate [NMDA] receptor subunit epsilon-2, also known as N-methyl D-aspartate receptor subtype 2B, is a protein that in humans is encoded by the GRIN2B gene.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.

The 5-HT7 receptor is a member of the GPCR superfamily of cell surface receptors and is activated by the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). The 5-HT7 receptor is coupled to Gs (stimulates the production of the intracellular signaling molecule cAMP) and is expressed in a variety of human tissues, particularly in the brain, the gastrointestinal tract, and in various blood vessels. This receptor has been a drug development target for the treatment of several clinical disorders. The 5-HT7 receptor is encoded by the HTR7 gene, which in humans is transcribed into 3 different splice variants.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.
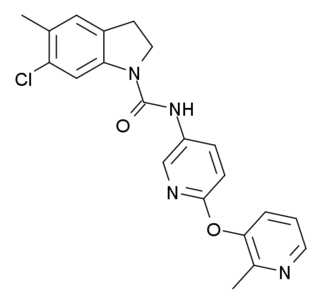
SB-242084 is a psychoactive drug and research chemical which acts as a selective antagonist for the 5HT2C receptor. It has anxiolytic effects, and enhances dopamine signalling in the limbic system, as well as having complex effects on the dopamine release produced by cocaine, increasing it in some brain regions but reducing it in others. It has been shown to increase the effectiveness of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants, and may also reduce their side effects. In animal studies, SB-242084 produced stimulant-type activity and reinforcing effects, somewhat similar to but much weaker than cocaine or amphetamines.

SB-258585 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist, with a Ki of 8.9nM. It is used in its 125I radiolabelled form to map the distribution of 5-HT6 receptors in the brain.

SB-399885 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist, with a Ki of 9.0nM. SB-399885 and other 5-HT6 antagonists show nootropic effects in animal studies, as well as antidepressant and anxiolytic effects which are comparable to and synergistic with drugs such as imipramine and diazepam, and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.

SB-357134 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist. SB-357134 and other 5-HT6 antagonists show nootropic effects in animal studies, and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.

RS-102221 is a drug developed by Hoffmann–La Roche, which was one of the first compounds discovered that acts as a potent and selective antagonist at the serotonin 5-HT2C receptor, with around 100× selectivity over the closely related 5-HT2A and 5-HT2B receptors. It has anxiolytic effects in animal studies, increases the effectiveness of SSRI antidepressants, and shows a complex interaction with cocaine, increasing some effects but decreasing others, reflecting a role for the 5-HT2C receptor in regulation of the dopamine signalling system in the brain.

SB-269970 is a drug and research chemical developed by GlaxoSmithKline used in scientific studies. It is believed to act as a selective 5-HT7 receptor antagonist (EC50 = 1.25 nM) (or possibly inverse agonist). A subsequent study in guinea pig at a concentration of 10 μM showed that it also blocks the α2-adrenergic receptor. The large difference in test concentrations however confirms the selectivity of SB-269970 for the 5-HT7 receptor.

Meclinertant (SR-48692) is a drug which acts as a selective, non-peptide antagonist at the neurotensin receptor NTS1, and was the first non-peptide antagonist developed for this receptor. It is used in scientific research to explore the interaction between neurotensin and other neurotransmitters in the brain, and produces anxiolytic, anti-addictive and memory-impairing effects in animal studies.
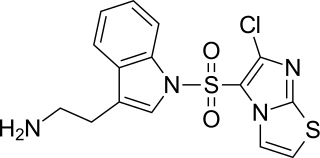
WAY-181187 is a high affinity and selective 5-HT6 receptor full agonist. It induces robust increases in extracellular GABA levels in the frontal cortex, hippocampus, striatum, and amygdala of rats without affecting concentrations in the nucleus accumbens or thalamus, and has modest to no effects on norepinephrine, serotonin, dopamine, or glutamate levels in these areas. WAY-181187 has demonstrated preclinical efficacy in rodent models of depression, anxiety, and notably obsessive-compulsive disorder, though it has also been shown to impair cognition and memory.
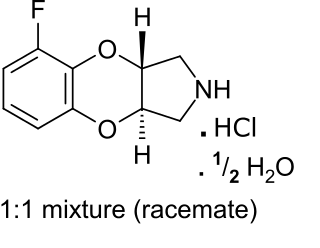
Fluparoxan is a potent α2-adrenergic receptor antagonist with excellent selectivity for this receptor over the α1-adrenergic receptor (2,630-fold), and is the only well-studied α2-adrenergic receptor antagonist in its structural family which does not antagonize any variant of the imidazoline receptor. It was shown to possess central α2-adrenoceptor antagonist activity after oral doses in man and was patented as an antidepressant by Glaxo in the early 1980s, but its development was discontinued when the compound failed to show a clear clinical advantage over existing therapies.
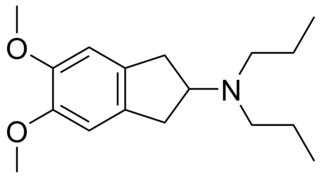
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.

SB-243213 is a research chemical which acts as a selective inverse agonist for the 5HT2C receptor and has anxiolytic effects. It has better than 100x selectivity for 5-HT2C over all other receptor subtypes tested, and a longer duration of action compared to older 5-HT2C antagonist ligands.




















