5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Azapirones are a class of drugs used as anxiolytics, antidepressants, and antipsychotics. They are commonly used as add-ons to other antidepressants, such as selective serotonin reuptake inhibitors (SSRIs).

Mianserin, sold under the brand name Tolvon among others, is an atypical antidepressant that is used primarily in the treatment of depression in Europe and elsewhere in the world. It is a tetracyclic antidepressant (TeCA). Mianserin is closely related to mirtazapine, both chemically and in terms of its actions and effects, although there are significant differences between the two drugs.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarization and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

5-Hydroxytryptamine (serotonin) receptor 5A, also known as HTR5A, is a protein that in humans is encoded by the HTR5A gene. Agonists and antagonists for 5-HT receptors, as well as serotonin uptake inhibitors, present promnesic (memory-promoting) and/or anti-amnesic effects under different conditions, and 5-HT receptors are also associated with neural changes.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

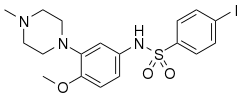
WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

2-Ethyl-5-methoxy-N,N-dimethyltryptamine (EMDT) is a tryptamine derivative which is used in scientific research. It acts as a selective 5-HT6 receptor agonist, with a Ki of 16 nM, and was one of the first selective agonists developed for this receptor. EMDT inhibits both short- and long-term memory formation in animal studies, and this effect can be reversed by the selective 5-HT6 antagonist SB-399,885. Additionally, it is active in the tail suspension test, suggesting that it could be an effective antidepressant.

SB-399885 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist, with a Ki of 9.0nM. SB-399885 and other 5-HT6 antagonists show nootropic effects in animal studies, as well as antidepressant and anxiolytic effects which are comparable to and synergistic with drugs such as imipramine and diazepam, and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.

SB-357134 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist. SB-357134 and other 5-HT6 antagonists show nootropic effects in animal studies, and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.

SB-271046 is a drug which is used in scientific research. It was one of the first selective 5-HT6 receptor antagonists to be discovered, and was found through high-throughput screening of the SmithKline Beecham Compound Bank using cloned 5-HT6 receptors as a target, with an initial lead compound being developed into SB-271046 through a structure-activity relationship (SAR) study. SB-271046 was found to be potent and selective in vitro and had good oral bioavailability in vivo, but had poor penetration across the blood–brain barrier, so further SAR work was then conducted, which led to improved 5-HT6 antagonists such as SB-357,134 and SB-399,885.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

RS-102221 is a drug developed by Hoffmann–La Roche, which was one of the first compounds discovered that acts as a potent and selective antagonist at the serotonin 5-HT2C receptor, with around 100× selectivity over the closely related 5-HT2A and 5-HT2B receptors. It has anxiolytic effects in animal studies, increases the effectiveness of SSRI antidepressants, and shows a complex interaction with cocaine, increasing some effects but decreasing others, reflecting a role for the 5-HT2C receptor in regulation of the dopamine signalling system in the brain.

Pruvanserin is a selective 5-HT2A receptor antagonist which was under development by Eli Lilly and Company for the treatment of insomnia. It was in phase II clinical trials in 2008 but appears to have been discontinued as it is no longer in the company's development pipeline. In addition to its sleep-improving properties, pruvanserin has also been shown to have antidepressant, anxiolytic, and working memory-enhancing effects in animal studies.

WAY-208466 is a potent and highly selective full agonist of the 5-HT6 receptor. It increases GABA levels in the cerebral cortex and tolerance does not appear to occur to this action upon chronic administration. Animal studies have shown that WAY-208466 produces antidepressant and anxiolytic effects in rodents and it may also be useful in the treatment of obsessive-compulsive disorder.
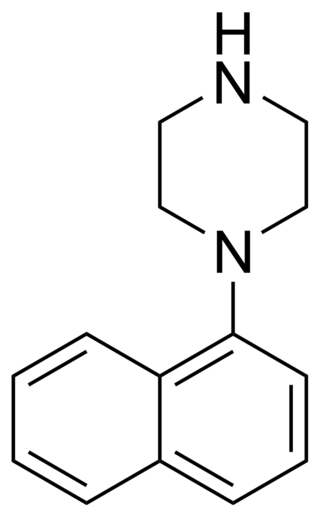
1-(1-Naphthyl)piperazine (1-NP) is a drug which is a phenylpiperazine derivative. It acts as a non-selective, mixed serotonergic agent, exerting partial agonism at the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F receptors, while antagonizing the 5-HT2A, 5-HT2B, and 5-HT2C receptors. It has also been shown to possess high affinity for the 5-HT3, 5-HT5A, 5-HT6, and 5-HT7 receptors, and may bind to 5-HT4 and the SERT as well. In animals it produces effects including hyperphagia, hyperactivity, and anxiolysis, of which are all likely mediated predominantly or fully by blockade of the 5-HT2C receptor.

MGS-0039 is a drug that is used in neuroscientific research, which acts as a potent and selective antagonist for group II of the metabotropic glutamate receptors (mGluR2/3). It produces antidepressant and anxiolytic effects in animal studies, and has been shown to boost release of dopamine and serotonin in specific brain areas. Research has suggested this may occur through a similar mechanism as that suggested for the similarly glutamatergic drug ketamine.