
The substantia nigra (SN) is a basal ganglia structure located in the midbrain that plays an important role in reward and movement. Substantia nigra is Latin for "black substance", reflecting the fact that parts of the substantia nigra appear darker than neighboring areas due to high levels of neuromelanin in dopaminergic neurons. Parkinson's disease is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta.

The dopamine receptor D4 is a dopamine D2-like G protein-coupled receptor encoded by the DRD4 gene on chromosome 11 at 11p15.5.

Dopaminergic means "related to dopamine", a common neurotransmitter. Dopaminergic substances or actions increase dopamine-related activity in the brain.

Dihydrexidine (DAR-0100) is a moderately selective full agonist at the dopamine D1 and D5 receptors. It has approximately 10-fold selectivity for D1 and D5 over the D2 receptor. Although dihydrexidine has some affinity for the D2 receptor, it has functionally selective (highly biased) D2 signaling, thereby explaining why it lacks D2 agonist behavioral qualities.
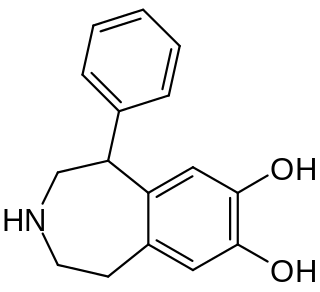
SKF-38393 is a synthetic compound of the benzazepine chemical class which acts as a selective D1/D5 receptor partial agonist. It has stimulant and anorectic effects.
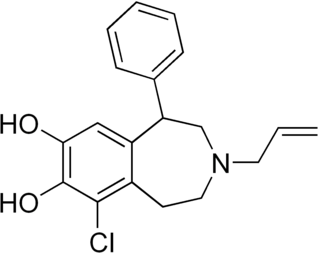
SKF-82,958 is a synthetic compound of the benzazepine class that acts as a D1/D5 receptor full agonist. SKF-82,958 and similar D1-like-selective full agonists like SKF-81,297 and 6-Br-APB produce characteristic anorectic effects, hyperactivity and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine. SKF-82,958 was also subsequently found to act as an agonist of ERα with negligible activity at ERβ, making it a subtype-selective estrogen.

Dopamine receptor D1, also known as DRD1. It is one of the two types of D1-like receptor family — receptors D1 and D5. It is a protein that in humans is encoded by the DRD1 gene.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.
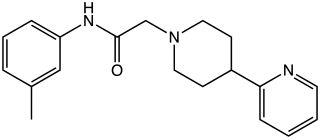
A-412,997 is a drug which acts as a dopamine agonist that is used in scientific research. It is the first drug developed that is a highly selective agonist for the D4 subtype, with significantly improved selectivity over older D4-preferring compounds such as PD-168,077 and CP-226,269. In animal tests it improved cognitive performance in rats to a similar extent as methylphenidate, but without producing place preference or other signs of abuse liability. Also unlike other dopamine agonists, selective D4 agonists do not cause side effects such as sedation and nausea, and so might have advantages over older dopamine agonist drugs.

6-Br-APB is a synthetic compound that acts as a selective D1 agonist, with the (R)-enantiomer being a potent full agonist, while the (S) enantiomer retains its D1 selectivity but is a weak partial agonist. (R)-6-Br-APB and similar D1-selective full agonists like SKF-81,297 and SKF-82,958 produce characteristic anorectic effects, stereotyped behaviour and self-administration in animals, with a similar but not identical profile to that of dopaminergic stimulants such as amphetamine.
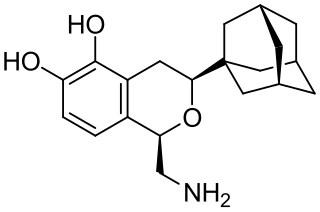
A-77636 is a synthetic drug which acts as a selective D1 receptor full agonist. It has nootropic, anorectic, rewarding and antiparkinsonian effects in animal studies, but its high potency and long duration of action causes D1 receptor downregulation and tachyphylaxis, and unlike other D1 full agonists such as SKF-82,958, it does not produce place preference in animals. A-77636 partially substituted for cocaine in animal studies, and has been suggested for use as a possible substitute drug in treating addiction, but it is better known for its use in studying the role of D1 receptors in the brain.

A-68930 is a synthetic compound that acts as a selective dopamine receptor D1 agonist. It is orally active and has antidepressant and anorectic effects in animals, producing wakefulness and tachycardia, but without stimulant effects, instead producing sedation. The difference in effects between A-68930 and other D1 agonists such as SKF-82958 may be due to their differing effects on the related D5 receptor.

7-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with reasonable selectivity for the D3 receptor subtype, and low affinity for serotonin receptors, unlike its structural isomer 8-OH-DPAT. 7-OH-DPAT is self-administered in several animal models, and is used to study its addiction effects to cocaine.

SKF-81,297 is a synthetic drug of the benzazepine chemical class that acts as a selective dopamine D1/D5 receptor full agonist, and produces a characteristic stimulant-like pattern of anorexia, hyperactivity and self-administration in animals. This profile is shared with several related drugs such as 6-Br-APB and SKF-82,958, but not with certain other D1 full agonists such as A-77,636, reflecting functional selectivity of D1 activation. Newer findings reveal that SKF-81,297 additionally acts as a partial agonist at D1-D2 receptor heteromers.
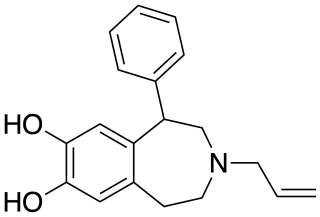
SKF-77,434 is a drug which acts as a selective dopamine D1 receptor partial agonist, and has stimulant and anorectic effects. Unlike other D1 agonists with higher efficacy such as SKF-81,297 and 6-Br-APB, SKF-77,434 does not maintain self-administration in animal studies, and so has been researched as a potential treatment for cocaine addiction.

Sumanirole (PNU-95,666) is a highly selective D2 receptor full agonist, the first of its kind to be discovered. It was developed for the treatment of Parkinson's disease and restless leg syndrome. While it has never been approved for medical use it is a highly valuable tool compound for basic research to identify neurobiological mechanisms that are based on a dopamine D2-linked (vs. D1-, D3-, D4-, and D5-linked) mechanism of action.
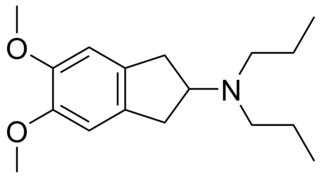
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

BP-897 is a drug used in scientific research which acts as a potent selective dopamine D3 receptor partial agonist with an in vitro intrinsic activity of ~0.6 and ~70x greater affinity for D3 over D2 receptors and is suspected to have partial agonist or antagonist activity in vivo. It has mainly been used in the study of treatments for cocaine addiction. A study comparing BP-897 with the potent, antagonistic, and highly D3 selective SB-277,011-A found, "SB 277011-A (1–10 mg/kg) was able to block cue-induced reinstatement of nicotine-seeking, indicating that DRD3 selective antagonism may be an effective approach to prevent relapse for nicotine. In contrast, BP 897 did not block the cue-induced reinstatement of nicotine-seeking or nicotine-taking under the FR5 schedule."

L-741,626 is a drug which acts as a potent and selective antagonist for the dopamine receptor D2. It has good selectivity over the related D3 and D4 subtypes and other receptors. L-741,626 is used for laboratory research into brain function and has proved particularly useful for distinguishing D2 mediated responses from those produced by the closely related D3 subtype, and for studying the roles of these subtypes in the action of cocaine and amphetamines in the brain.
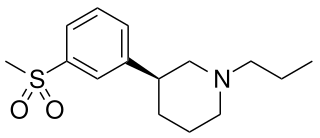
OSU-6162 (PNU-96391) is a compound which acts as a partial agonist at both dopamine D2 receptors and 5-HT2A receptors. It acts as a dopamine stabilizer in a similar manner to the closely related drug pridopidine, and has antipsychotic, anti-addictive and anti-Parkinsonian effects in animal studies. Both enantiomers show similar activity but with different ratios of effects, with the (S) enantiomer (–)-OSU-6162 that is more commonly used in research, having higher binding affinity to D2 but is a weaker partial agonist at 5-HT2A, while the (R) enantiomer (+)-OSU-6162 has higher efficacy at 5-HT2A but lower D2 affinity.




















