Pramipexole, sold under the brand Mirapex among others, is a medication used to treat Parkinson's disease (PD) and restless legs syndrome (RLS). In Parkinson's disease it may be used alone or together with levodopa. It is taken by mouth. Pramipexole is a dopamine agonist of the non-ergoline class.

Rotigotine, sold under the brand name Neupro among others, is a dopamine agonist of the non-ergoline class of medications indicated for the treatment of Parkinson's disease and restless legs syndrome. It is formulated as a once-daily transdermal patch which provides a slow and constant supply of the drug over the course of 24 hours.

Piribedil (trade names Pronoran, Trivastal Retard, Trastal, Trivastan, Clarium and others) is an antiparkinsonian agent and piperazine derivative which acts as a D2 and D3 receptor agonist. It also has α2-adrenergic antagonist properties.
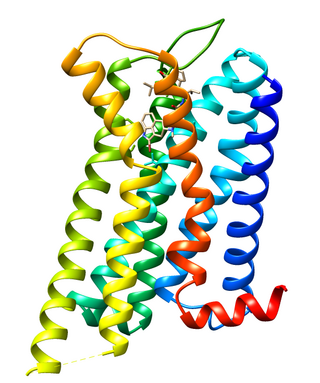
Dopamine receptor D2, also known as D2R, is a protein that, in humans, is encoded by the DRD2 gene. After work from Paul Greengard's lab had suggested that dopamine receptors were the site of action of antipsychotic drugs, several groups, including those of Solomon H. Snyder and Philip Seeman used a radiolabeled antipsychotic drug to identify what is now known as the dopamine D2 receptor. The dopamine D2 receptor is the main receptor for most antipsychotic drugs. The structure of DRD2 in complex with the atypical antipsychotic risperidone has been determined.

SB-277,011A is a drug which acts as a potent and selective dopamine D3 receptor antagonist, which is around 80–100 times selective for D3 over D2, and lacks any partial agonist activity.

Dopamine receptor D3 is a protein that in humans is encoded by the DRD3 gene.

GBR-12935 is a piperazine derivative which is a potent and selective dopamine reuptake inhibitor. It was originally developed in its 3H radiolabelled form for the purpose of mapping the distribution of dopaminergic neurons in the brain by selective labelling of dopamine transporter proteins. This has led to potential clinical uses in the diagnosis of Parkinson's disease, although selective radioligands such as Ioflupane (123I) are now available for this application. GBR-12935 is now widely used in animal research into Parkinson's disease and the dopamine pathways in the brain.

UH-232 ((+)-UH232) is a drug which acts as a subtype selective mixed agonist-antagonist for dopamine receptors, acting as a weak partial agonist at the D3 subtype, and an antagonist at D2Sh autoreceptors on dopaminergic nerve terminals. It causes dopamine release in the brain and has a stimulant effect, as well as blocking the behavioural effects of cocaine. It may also serve as a 5-HT2A receptor agonist, based on animal studies. It was investigated in clinical trials for the treatment of schizophrenia, but unexpectedly caused symptoms to become worse.

AS-8112 is a synthetic compound that acts as a selective antagonist at the dopamine receptor subtypes D2 and D3, and the serotonin receptor 5-HT3. It has potent antiemetic effects in animal studies and has been investigated for potential medical use.

7-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with reasonable selectivity for the D3 receptor subtype, and low affinity for serotonin receptors, unlike its structural isomer 8-OH-DPAT. 7-OH-DPAT is self-administered in several animal models, and is used to study its addiction effects to cocaine.

PF-219,061 is a drug that was under development by Pfizer which acts as a potent and highly selective agonist for the dopamine D3 receptor. It was under development as a potential medication for the treatment of female sexual dysfunction. It did not advance into clinical trials.

8-OH-PBZI is a drug used in scientific research which acts as a potent and selective agonist for the dopamine D3 receptor.

PD-128,907 is a drug used in scientific research which acts as a potent and selective agonist for the dopamine D2 and D3 receptors. It is used for studying the role of these receptors in the brain, in roles such as inhibitory autoreceptors that act to limit further dopamine release, as well as release of other neurotransmitters. In animal studies, it has been shown to reduce toxicity from cocaine overdose.
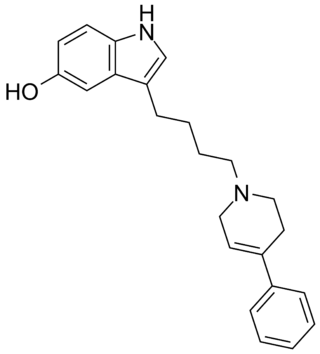
Roxindole (EMD-49,980) is a dopaminergic and serotonergic drug which was originally developed by Merck KGaA for the treatment of schizophrenia. In clinical trials its antipsychotic efficacy was only modest but it was unexpectedly found to produce potent and rapid antidepressant and anxiolytic effects. As a result, roxindole was further researched for the treatment of depression instead. It has also been investigated as a therapy for Parkinson's disease and prolactinoma.
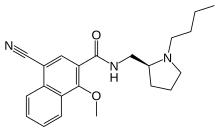
PNU-99,194(A) (or U-99,194(A)) is a drug which acts as a moderately selective D3 receptor antagonist with ~15-30-fold preference for D3 over the D2 subtype. Though it has substantially greater preference for D3 over D2, the latter receptor does still play some role in its effects, as evidenced by the fact that PNU-99,194 weakly stimulates both prolactin secretion and striatal dopamine synthesis, actions it does not share with the more selective (100-fold) D3 receptor antagonists S-14,297 and GR-103,691.

BP-897 is a drug used in scientific research which acts as a potent selective dopamine D3 receptor partial agonist with an in vitro intrinsic activity of ~0.6 and ~70x greater affinity for D3 over D2 receptors and is suspected to have partial agonist or antagonist activity in vivo. It has mainly been used in the study of treatments for cocaine addiction. A study comparing BP-897 with the potent, antagonistic, and highly D3 selective SB-277,011-A found, "SB 277011-A (1–10 mg/kg) was able to block cue-induced reinstatement of nicotine-seeking, indicating that DRD3 selective antagonism may be an effective approach to prevent relapse for nicotine. In contrast, BP 897 did not block the cue-induced reinstatement of nicotine-seeking or nicotine-taking under the FR5 schedule."

L-741,626 is a drug which acts as a potent and selective antagonist for the dopamine receptor D2. It has good selectivity over the related D3 and D4 subtypes and other receptors. L-741,626 is used for laboratory research into brain function and has proved particularly useful for distinguishing D2 mediated responses from those produced by the closely related D3 subtype, and for studying the roles of these subtypes in the action of cocaine and amphetamines in the brain.

PF-592,379 is a drug developed by Pfizer which acts as a potent, selective and orally active agonist for the dopamine D3 receptor, which was under development as a potential medication for the treatment of female sexual dysfunction and male erectile dysfunction. Unlike some other less selective D3 agonists, a research study showed that PF-592,379 has little abuse potential in animal studies, and so was selected for further development and potentially human clinical trials. Development has since been discontinued.

Tavapadon is a dopamine receptor agonist which is under development for the treatment of Parkinson's disease. It is under development by Cerevel Therapeutics, which acquired tavapadon from Pfizer in 2018. It is taken by mouth.

Trazpiroben (developmental code name TAK-906) is a dopamine antagonist drug which was under development for the treatment of gastroparesis. It acts as a peripherally selective dopamine D2 and D3 receptor antagonist. The drug has been found to strongly increase prolactin levels in humans, similarly to other peripherally selective D2 receptor antagonists like domperidone. Clinical development of trazpiroben was discontinued before April 2022. Trazpiroben was originated by Altos Therapeutics and was under development by Takeda Oncology.