In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Ethanethiol, commonly known as ethyl mercaptan, is an organosulfur compound with the formula CH3CH2SH. is a colorless liquid with a distinct odor. Abbreviated EtSH, it consists of an ethyl group (Et), CH3CH2, attached to a thiol group, SH. Its structure parallels that of ethanol, but with sulfur in place of oxygen. The odor of EtSH is infamous. Ethanethiol is more volatile than ethanol due to a diminished ability to engage in hydrogen bonding. Ethanethiol is toxic in high concentrations. It occurs naturally as a minor component of petroleum, and may be added to otherwise odorless gaseous products such as liquefied petroleum gas (LPG) to help warn of gas leaks. At these concentrations, ethanethiol is not harmful.

Diethyl ether, or simply ether, is an organic compound in the ether class with the formula C4H10O, (CH3CH2)2O or (C2H5)2O, sometimes abbreviated as Et2O. It is a colourless, highly volatile, sweet-smelling, extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication.

Hermann Emil Louis Fischer was a German chemist and 1902 recipient of the Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of drawing asymmetric carbon atoms. He also hypothesized lock and key mechanism of enzyme action. He never used his first given name, and was known throughout his life simply as Emil Fischer.

Amobarbital is a drug that is a barbiturate derivative. It has sedative-hypnotic properties. It is a white crystalline powder with no odor and a slightly bitter taste. It was first synthesized in Germany in 1923. It is considered a short to intermediate acting barbiturate. If amobarbital is taken for extended periods of time, physiological and psychological dependence can develop. Amobarbital withdrawal mimics delirium tremens and may be life-threatening. Amobarbital was manufactured by Eli Lilly and Company in the US under the brand name Amytal in bright blue bullet shaped capsules or pink tablets containing 50, 100, or 200 milligrams of the drug. The drug was also manufactured generically. Amobarbital was widely misused, known as "Blue Heavens" on the street. Amytal, as well as Tuinal, a combination drug containing equal quantities of secobarbital and amobarbital, were both manufactured by Eli Lilly until the late-1990s. However, as the popularity of benzodiazepines increased, prescriptions for these medications became increasingly rare beginning in the mid to late-1980s.

Phenobarbital, also known as phenobarbitone or phenobarb, sold under the brand name Luminal among others, is a medication of the barbiturate type. It is recommended by the World Health Organization (WHO) for the treatment of certain types of epilepsy in developing countries. In the developed world, it is commonly used to treat seizures in young children, while other medications are generally used in older children and adults. In developed countries it is used for veterinary purposes. It may be used intravenously, injected into a muscle, or taken by mouth. The injectable form may be used to treat status epilepticus. Phenobarbital is occasionally used to treat trouble sleeping, anxiety, and drug withdrawal and to help with surgery. It usually begins working within five minutes when used intravenously and half an hour when administered by mouth. Its effects last for between four hours and two days.
Barbituric acid or malonylurea or 6-hydroxyuracil is an organic compound based on a pyrimidine heterocyclic skeleton. It is an odorless powder soluble in water. Barbituric acid is the parent compound of barbiturate drugs, although barbituric acid itself is not pharmacologically active. The compound was first synthesised by Adolf von Baeyer.

Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH3CH2−O−C(=O)−N=N−C(=O)−O−CH2CH3. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles.
Pentobarbital (US) or pentobarbitone is a short-acting barbiturate typically used as a sedative, a preanesthetic, and to control convulsions in emergencies. It can also be used for short-term treatment of insomnia but has been largely replaced by the benzodiazepine family of drugs.
Chloral, also known as trichloroacetaldehyde or trichloroethanal, is the organic compound with the formula Cl3CCHO. This aldehyde is a colourless liquid that is soluble in a wide range of solvents. It reacts with water to form chloral hydrate, a once widely used sedative and hypnotic substance.

Ethyl sulfate, also known as sulfovinic acid, is an organic chemical compound used as an intermediate in the production of ethanol from ethylene. It is the ethyl ester of sulfuric acid.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).
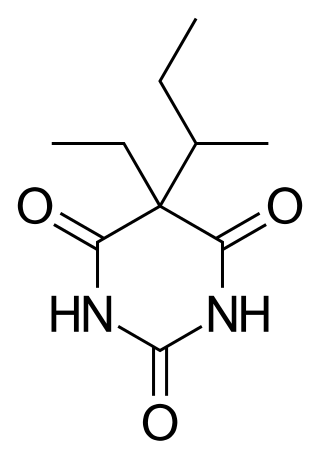
Butabarbital is a prescription barbiturate sleep aid and anxiety medication. Butabarbital has a particularly fast onset of effects and short duration of action compared to other barbiturates, which makes it useful for certain applications such as treating severe insomnia, relieving general anxiety and relieving anxiety before surgical procedures; however it is also relatively dangerous particularly when combined with alcohol, and so is now rarely used, although it is still prescribed in some Eastern European and South American countries. Its intermediate duration of action gives butabarbital an abuse potential slightly lower than secobarbital. Butabarbital can be hydrolyzed to valnoctamide.

Josef, Baron von Mering was a German physician.

In chemistry, an adduct is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species. Examples include the addition of sodium bisulfite to an aldehyde to give a sulfonate. It can be considered as a single product resulting from the direct combination of different molecules which comprises all atoms of the reactant molecules.
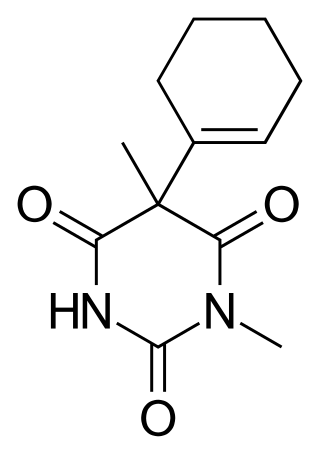
Hexobarbital or hexobarbitone, sold both in acid and sodium salt forms as Citopan, Evipan, and Tobinal, is a barbiturate derivative having hypnotic and sedative effects. It was used in the 1940s and 1950s as an agent for inducing anesthesia for surgery, as well as a rapid-acting, short-lasting hypnotic for general use, and has a relatively fast onset of effects and short duration of action. Modern barbiturates have largely supplanted the use of hexobarbital as an anesthetic, as they allow for better control of the depth of anesthesia. Hexobarbital is still used in some scientific research.

Sulfonmethane is a chemical compound first synthesized by Eugen Baumann in 1888 and introduced as a hypnotic drug by Alfred Kast later on, but now superseded by newer and safer sedatives. Its appearance is either in colorless crystalline or powdered form. In United States, it is scheduled as a Schedule III drug in the Controlled Substance Act.

Barbiturates are a class of depressant drugs that are chemically derived from barbituric acid. They are effective when used medically as anxiolytics, hypnotics, and anticonvulsants, but have physical and psychological addiction potential as well as overdose potential among other possible adverse effects. They have been used recreationally for their anti-anxiety and sedative effects, and are thus controlled in most countries due to the risks associated with such use.
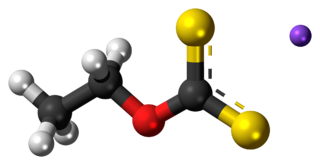
Sodium ethyl xanthate (SEX) is an organosulfur compound with the chemical formula CH3CH2OCS2Na. It is a pale yellow powder, which is usually obtained as the dihydrate. Sodium ethyl xanthate is used in the mining industry as a flotation agent. A closely related potassium ethyl xanthate (KEX) is obtained as the anhydrous salt.

Phenylsodium C6H5Na is an organosodium compound. Solid phenylsodium was first isolated by Nef in 1903. Although the behavior of phenylsodium and phenyl magnesium bromide are similar, the organosodium compound is very rarely used.