
Alprazolam, sold under the brand name Xanax, is a fast-acting, potent tranquilizer of moderate duration within the triazolobenzodiazepine group of chemicals called benzodiazepines. Alprazolam is most commonly used in management of anxiety disorders, specifically panic disorder or generalized anxiety disorder (GAD). Other uses include the treatment of chemotherapy-induced nausea, together with other treatments. GAD improvement occurs generally within a week. Alprazolam is generally taken orally.

Nitrazepam, sold under the brand name Mogadon among others, is a hypnotic drug of the benzodiazepine class used for short-term relief from severe, disabling anxiety and insomnia. It also has sedative (calming) properties, as well as amnestic, anticonvulsant, and skeletal muscle relaxant effects.

Bromazepam, sold under many brand names, is a benzodiazepine. It is mainly an anti-anxiety agent with similar side effects to diazepam. In addition to being used to treat anxiety or panic states, bromazepam may be used as a premedicant prior to minor surgery. Bromazepam typically comes in doses of 3 mg and 6 mg tablets.
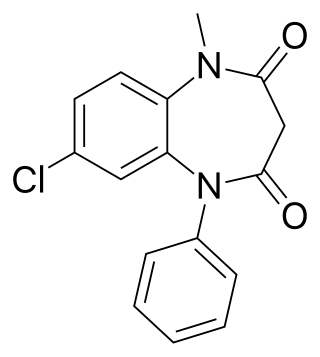
Clobazam, sold under the brand names Frisium, Onfi and others, is a benzodiazepine class medication that was patented in 1968. Clobazam was first synthesized in 1966 and first published in 1969. Clobazam was originally marketed as an anxioselective anxiolytic since 1970, and an anticonvulsant since 1984. The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.

Nordazepam is a 1,4-benzodiazepine derivative. Like other benzodiazepine derivatives, it has amnesic, anticonvulsant, anxiolytic, muscle relaxant, and sedative properties. However, it is used primarily in the treatment of anxiety disorders. It is an active metabolite of diazepam, chlordiazepoxide, clorazepate, prazepam, pinazepam, and medazepam.

Prazepam is a benzodiazepine derivative drug developed by Warner-Lambert in the 1960s. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Prazepam is a prodrug for desmethyldiazepam which is responsible for the therapeutic effects of prazepam.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Etizolam is a thienodiazepine derivative which is a benzodiazepine analog. The etizolam molecule differs from a benzodiazepine in that the benzene ring has been replaced by a thiophene ring and triazole ring has been fused, making the drug a thienotriazolodiazepine.
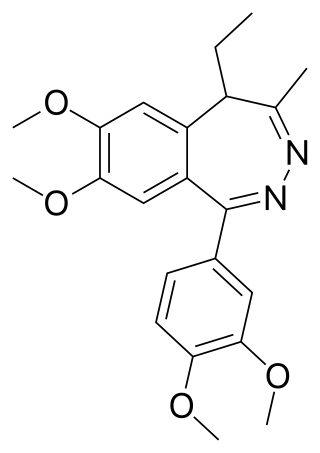
Tofisopam is an anxiolytic that is marketed in several European countries. Chemically, it is a 2,3-benzodiazepine. Unlike other anxiolytic benzodiazepines however, tofisopam does not have anticonvulsant, sedative, skeletal muscle relaxant, motor skill-impairing or amnestic properties. While it may not be an anticonvulsant in and of itself, it has been shown to enhance the anticonvulsant action of classical 1,4-benzodiazepines and muscimol, but not sodium valproate, carbamazepine, phenobarbital, or phenytoin. Tofisopam is indicated for the treatment of anxiety and alcohol withdrawal, and is prescribed in a dosage of 50–300 mg per day divided into three doses. Peak plasma levels are attained two hours after an oral dose. Tofisopam is not reported as causing dependence to the same extent as other benzodiazepines, but is still recommended to be prescribed for a maximum of 12 weeks.
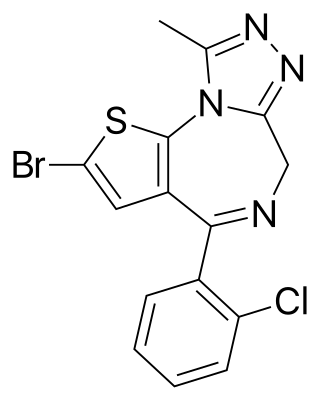
Brotizolam is a sedative-hypnotic thienotriazolodiazepine drug which is a benzodiazepine analog. It possesses anxiolytic, anticonvulsant, hypnotic, sedative and skeletal muscle relaxant properties, and is considered to be similar in effect to other short-acting hypnotic benzodiazepines such as triazolam or midazolam. It is used in the short-term treatment of severe insomnia. Brotizolam is a highly potent and short-acting hypnotic, with a typical dose ranging from 0.125 to 0.25 milligrams, which is rapidly eliminated with an average half-life of 4.4 hours.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.

DMCM is a drug from the β-carboline family that induces anxiety and convulsions by acting as a negative allosteric modulator of GABAA receptors — functionally opposite to benzodiazepines and related drugs which are positive allosteric modulators — and is used in scientific research for these properties to test new anxiolytic and anticonvulsant medications, respectively. It has also been shown to produce analgesic effects in animals, which is thought to be the drug's induced panic reducing the perception of pain.

Zapizolam is a pyridodiazepine drug, which is a benzodiazepine analog of pyridotriazolodiazepine group. It has sedative and anxiolytic effects similar to those produced by benzodiazepine derivatives, and has been sold illicitly as a designer drug.

Rilmazafone is a water-soluble prodrug developed in Japan. Once metabolized, rilmazafone is converted into several benzodiazepine metabolites that have sedative and hypnotic effects. These metabolites induce impairment of motor function and have hypnotic properties.

Tifluadom is a benzodiazepine derivative with an unusual activity profile. Unlike most benzodiazepines, tifluadom has no activity at the GABAA receptor, but instead is a selective agonist for the κ-opioid receptor. It has potent analgesic and diuretic effects in animals, and also has sedative effects and stimulates appetite.

Wogonin is an O-methylated flavone, a flavonoid-like chemical compound which is found in Scutellaria baicalensis.

CP-1414S is an experimental drug first made by a team in Germany. It is a benzodiazepine derivative. CP-1414S is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring, and so is most closely related to other 1,5-benzodiazepines such as clobazam.

A thienotriazolodiazepine is a heterocyclic compound containing a diazepine ring fused to thiophene and triazole rings. Thienotriazolodiazepine forms the central core of several pharmaceutical drugs including:
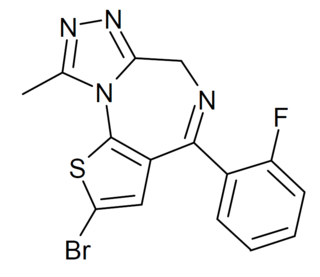
Flubrotizolam is a thienotriazolodiazepine derivative with potent sedative and anxiolytic effects, which has been sold as a designer drug.

Apafant is a drug which acts as a potent and selective inhibitor of the phospholipid mediator platelet-activating factor (PAF). It was developed by structural modification of the thienotriazolodiazepine sedative drug brotizolam and demonstrated that PAF inhibitory actions could be separated from activity at the benzodiazepine receptor. Apafant was investigated for several applications involving inflammatory responses such as asthma and conjunctivitis but was never adopted for medical use, however it continues to be used in pharmacology research.





















