Etizolam is a thienodiazepine derivative which is a benzodiazepine analog. The etizolam molecule differs from a benzodiazepine in that the benzene ring has been replaced by a thiophene ring and triazole ring has been fused, making the drug a thienotriazolodiazepine.

Phenazepam is a benzodiazepine drug, which was developed in the Soviet Union in 1975, and now produced in Russia and some affiliated countries.

Substituted cathinones, which include some stimulants and entactogens, are derivatives of cathinone. They feature a phenethylamine core with an alkyl group attached to the alpha carbon, and a ketone group attached to the beta carbon, along with additional substitutions. Cathinone occurs naturally in the plant khat whose leaves are chewed as a recreational drug.

Ro5-4864 (4'-chlorodiazepam) is a drug which is a benzodiazepine derivative of diazepam. However unlike most benzodiazepine derivatives, Ro5-4864 lacks affinity for GABAA receptors and lacks typical benzodiazepine effects, instead being sedative yet also convulsant and anxiogenic in effects. Ro5-4864 was found to be a potent ligand for the "peripheral benzodiazepine receptor", later renamed to mitochondrial translocator protein 18kDa (TSPO). Despite its convulsant effects, at lower doses Ro5-4864 has proved to be neuroprotective and has become widely used for research into the role of the TSPO protein in neurotoxicity. In vitro studies and rodent models also suggest the possibility of analgesic, antidepressant, cardioprotective, and anti-cancer effects.
N-Desalkylflurazepam is a benzodiazepine analog and an active metabolite of several other benzodiazepine drugs including flurazepam, flutoprazepam, fludiazepam, midazolam, flutazolam, quazepam, and ethyl loflazepate. It is long-acting, prone to accumulation, and binds unselectively to the various benzodiazepine receptor subtypes. It has been sold as a designer drug from 2016 onward.

Pyrazolam (SH-I-04) is a benzodiazepine derivative originally developed by a team led by Leo Sternbach at Hoffman-La Roche in the 1970s. It has since been "rediscovered" and sold as a designer drug since 2012.

Diclazepam (Ro5-3448), also known as chlorodiazepam and 2'-chloro-diazepam, is a benzodiazepine and functional analog of diazepam. It was first synthesized by Leo Sternbach and his team at Hoffman-La Roche in 1960. It is not currently approved for use as a medication, but rather sold as an unscheduled substance. Efficacy and safety have not been tested in humans.
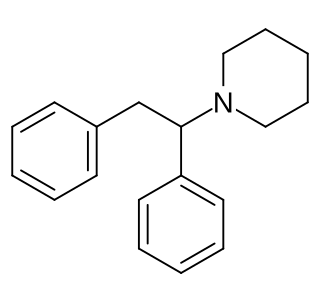
Diphenidine is a dissociative anesthetic that has been sold as a designer drug. The synthesis of diphenidine was first reported in 1924, and employed a Bruylants reaction analogous to the one that would later be used to discover phencyclidine in 1956. Shortly after the 2013 UK ban on arylcyclohexylamines, diphenidine and the related compound methoxphenidine became available on the grey market. Anecdotal reports describe high doses of diphenidine producing "bizarre somatosensory phenomena and transient anterograde amnesia." Diphenidine and related diarylethylamines have been studied in vitro as treatments for neurotoxic injury and are antagonists of the NMDA receptor. In dogs diphenidine exhibits greater antitussive potency than codeine phosphate.

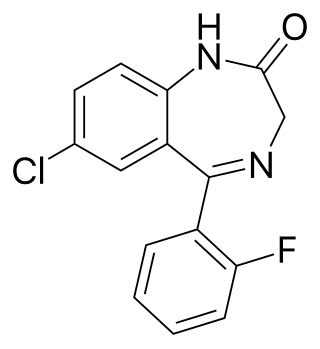
Flubromazepam is a benzodiazepine derivative which was first synthesized in 1960, but was never marketed and did not receive any further attention or study until late 2012 when it appeared on the grey market as a novel designer drug.

3-Hydroxyphenazepam is a benzodiazepine with hypnotic, sedative, anxiolytic, and anticonvulsant properties. It is an active metabolite of phenazepam, as well as the active metabolite of the benzodiazepine prodrug cinazepam. Relative to phenazepam, 3-hydroxyphenazepam has diminished myorelaxant properties, but is about equivalent in most other regards. Like other benzodiazepines, 3-hydroxyphenazepam behaves as a positive allosteric modulator of the benzodiazepine site of the GABAA receptor with an EC50 value of 10.3 nM. It has been sold online as a designer drug.

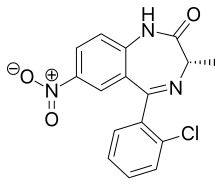
Clonazolam is a drug of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It has had very little research done about its effects and metabolism, and has been sold online as a designer drug.
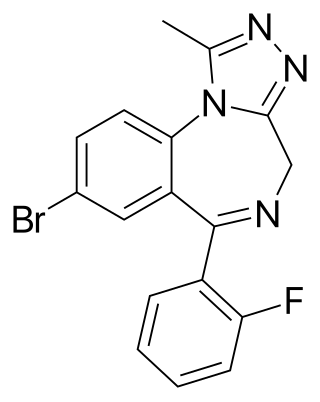
Flubromazolam (JYI-73) is a triazolobenzodiazepine (TBZD), which are a benzodiazepine (BZD) chemical subclass. Flubromazolam is reputed to be highly potent, and concerns have been raised that clonazolam and flubromazolam in particular may pose comparatively higher risks than other designer benzodiazepines, due to their ability to produce strong sedation and amnesia at oral doses of as little as 0.5 mg. Life-threatening adverse reactions have been observed at doses of only 3 mg of flubromazolam. Currently, most black market benzodiazepines being distributed illegally are alprazolam, clonazepam, or research chemical benzodiazepines. Oftentimes, with clonazepam and the non-alprazolam benzodiazepines coming in the form of counterfeit pressed pills such as the blue-colored B707’s or green S903’s; with the latter having often been observed as being a mixture of both clonazepam and flubromazolam. Flubromazolam however, having a much longer duration and powerful debilitating effects on motor control and physical coordination. Flubromazolam may also be a favorable admixture for illegal pressed pills due to it showing no reaction on the “TN Scientific BZD” testing reagent.
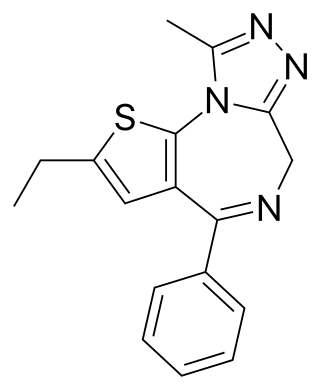
Deschloroetizolam is a thienotriazolodiazepine that is the dechlorinated analog of the closely related etizolam. The compound has been sold as a designer drug.
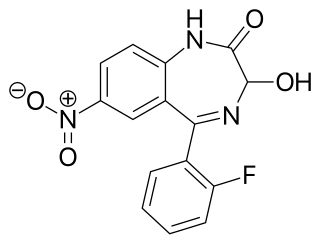
Nifoxipam is a benzodiazepine that is a minor metabolite of flunitrazepam and has been sold online as a designer drug.

Nitrazolam is a triazolobenzodiazepine (TBZD) , which are benzodiazepine (BZD) derivatives, that has been sold online as a designer drug.

Bromazolam (XLI-268) is a triazolobenzodiazepine (TBZD) which was first synthesised in 1976, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified by the EMCDDA in Sweden in 2016. It is the bromo instead of chloro analogue of alprazolam and has similar sedative and anxiolytic effects to it and other benzodiazepines. Bromazolam is a non subtype selective agonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 2.81nM at the α1 subtype, 0.69nM at α2 and 0.62nM at α5.

Flualprazolam is a tranquilizer of the triazolobenzodiazepine (TBZD) class, which are benzodiazepines (BZDs) fused with a triazole ring. It was first synthesised in 1976, but was never marketed. It can be seen as the triazolo version of fludiazepam. It has subsequently been sold as a designer drug, first being definitively identified as such in Sweden in 2018. It can be described as the 2'-fluoro derivative of alprazolam or the fluoro instead of chloro analogue of triazolam, and has similar sedative and anxiolytic effects.

Fluclotizolam is a thienotriazolodiazepine derivative which was first synthesised in 1979, but was never marketed. It has subsequently been sold as a designer drug, first being definitively identified in 2017.
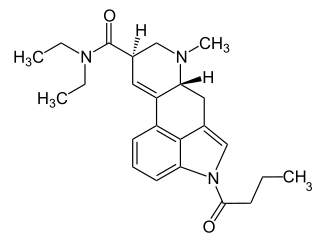
1B-LSD is an acylated derivative of lysergic acid diethylamide (LSD), which has been sold as a designer drug. In tests on mice it was found to be an active psychedelic, though with only around 1/7 the potency of LSD itself.