Phenothiazine, abbreviated PTZ, is an organic compound that has the formula S(C6H4)2NH and is related to the thiazine-class of heterocyclic compounds. Derivatives of phenothiazine are highly bioactive and have widespread use and rich history. The derivatives chlorpromazine and promethazine revolutionized the fields of psychiatry and allergy treatment, respectively. An earlier derivative, methylene blue, was one of the first antimalarial drugs, and derivatives are under investigation as possible anti-infective drugs. Phenothiazine is a prototypical pharmaceutical lead structure in medicinal chemistry.

Fluacizine, sold under the brand name Phtorazisin, is a tricyclic antidepressant (TCA) of the phenothiazine group which is or was marketed in Russia. Unlike other phenothiazines, fluacizine is not an antipsychotic, and can actually reverse catalepsy and extrapyramidal symptoms induced by antidopaminergic agents like antipsychotics, reserpine, and tetrabenazine as well as potentiate amphetamine-induced stereotypy. It is known to act as a norepinephrine reuptake inhibitor, antihistamine, and anticholinergic. The drug was developed in the 1960s and was marketed in the 1970s. It is the trifluoromethyl analogue of chloracizine.

Oxatomide, sold under the brand name Tinset among others, is a first-generation antihistamine of the diphenylmethylpiperazine family which is marketed in Europe, Japan, and a number of other countries. It was discovered at Janssen Pharmaceutica in 1975. Oxatomide lacks any anticholinergic effects. In addition to its H1 receptor antagonism, it also possesses antiserotonergic activity similarly to hydroxyzine.

Piritramide(R-3365, trade names Dipidolor, Piridolan, Pirium and others) is a synthetic opioid analgesic that is marketed in certain European countries including: Austria, Belgium, Czech Republic, Slovenia, Germany and the Netherlands. It comes in free form, is about 0.75x times as potent as morphine and is given parenterally for the treatment of severe pain. Nausea, vomiting, respiratory depression and constipation are believed to be less frequent with piritramide than with morphine, and it produces more rapid-onset analgesia when compared to morphine and pethidine. After intravenous administration the onset of analgesia is as little as 1–2 minutes, which may be related to its great lipophilicity. The analgesic and sedative effects of piritramide are believed to be potentiated with phenothiazines and its emetic (nausea/vomiting-inducing) effects are suppressed. The volume of distribution is 0.7-1 L/kg after a single dose, 4.7-6 L/kg after steady-state concentrations are achieved and up to 11.1 L/kg after prolonged dosing.

Mibolerone, also known as dimethylnortestosterone (DMNT) and sold under the brand names Cheque Drops and Matenon, is a synthetic, orally active, and extremely potent anabolic–androgenic steroid (AAS) and a 17α-alkylated nandrolone (19-nortestosterone) derivative which was marketed by Upjohn for use as a veterinary drug. It was indicated specifically as an oral treatment for prevention of estrus (heat) in adult female dogs.

Etymemazine is an antipsychotic, antihistamine and anticholinergic drug of the phenothiazine chemical class, structurally related to cyamemazine and methotrimeprazine.

Fluanisone is a typical antipsychotic and sedative of the butyrophenone chemical class. It is used in the treatment of schizophrenia and mania. It is also a component of the injectable veterinary formulation fentanyl/fluanisone (Hypnorm) where it is used for rodent analgesia during short surgical procedures. This fentanyl/fluanisone combination is known as a so-called neuroleptanalgesic.

Chlorproethazine, sold under the brand name Neuriplege, is a drug of the phenothiazine group described as a muscle relaxant or tranquilizer which is or has been marketed in Europe as a topical cream for the treatment of muscle pain. It has been associated with photoallergic contact dermatitis.

Lorpiprazole (INN) is a marketed anxiolytic drug of the phenylpiperazine group. It has been described as a serotonin antagonist and reuptake inhibitor (SARI) in the same group as trazodone, nefazodone, and etoperidone.

Tolpiprazole is an anxiolytic drug of the phenylpiperazine group that was never marketed.
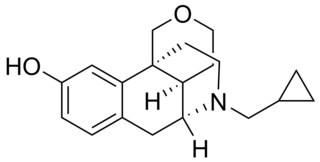
Proxorphan (INN), also known as proxorphan tartate (USAN), is an opioid analgesic and antitussive drug of the morphinan family that was never marketed. It acts preferentially as a κ-opioid receptor partial agonist and to a lesser extent as a μ-opioid receptor partial agonist.

Pipamazine is a drug of the phenothiazine class formerly used as an antiemetic. It is chemically related to chlorpromazine, but has negligible antipsychotic activity and produces few extrapyramidal side effects.

Glemanserin (INN) is a drug which acts as a potent and selective 5-HT2A receptor antagonist. The first truly selective 5-HT2A ligand to be discovered, glemanserin resulted in the development of the widely used and even more potent and selective 5-HT2A receptor antagonist volinanserin (MDL-100,907), which is a fluorinated analogue. Though it was largely superseded in scientific research by volinanserin, glemanserin was investigated clinically for the treatment of generalized anxiety disorder. However, it was ultimately found to be ineffective and was not marketed.

Phenbenzamine, sold under the brand name Antergan and known by the former developmental code name RP-2339, is an antihistamine of the ethylenediamine class which also has anticholinergic properties. It was introduced in 1941 or 1942 and was the first antihistamine to be introduced for medical use. Soon following its introduction, phenbenzamine was replaced by another antihistamine of the same class known as mepyramine. Following this, other antihistamines, such as diphenhydramine, promethazine, and tripelennamine, were developed and introduced. Owing to their sedative effects, phenbenzamine and promethazine were assessed in the treatment of manic depression in France in the 1940s and were regarded as promising therapies for such purposes. Whereas phenbenzamine was the first clinically useful antihistamine, piperoxan was the first compound with antihistamine properties to be discovered and was synthesized in the early 1930s.
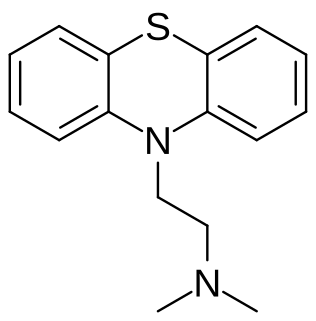
Fenethazine (INN), or phenethazine, is a first-generation antihistamine of the phenothiazine group. Promethazine, and subsequently chlorpromazine, were derived from fenethazine. Fenethazine, in turn, was derived from phenbenzamine.
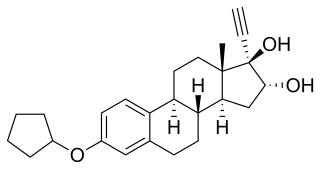
Nilestriol (INN), also known as nylestriol, is a synthetic estrogen which was patented in 1971 and is marketed in China. It is the 3-cyclopentyl ether of ethinylestriol, and is also known as ethinylestriol cyclopentyl ether (EE3CPE). Nilestriol is a prodrug of ethinylestriol, and is a more potent estrogen in comparison. It is described as a slowly-metabolized, long-acting estrogen and derivative of estriol. Nilestriol was assessed in combination with levonorgestrel for the potential treatment of postmenopausal osteoporosis, but this formulation ultimately was not marketed.
Triparanol was the first synthetic cholesterol-lowering drug. It was patented in 1959 and introduced in the United States in 1960. The developmental code name of triparanol, MER/29, became so well known that it became the registered trade name of the drug. It was withdrawn in 1962 due to severe adverse effects such as nausea and vomiting, vision loss due to irreversible cataracts, alopecia, skin disorders, and accelerated atherosclerosis. It is now considered to be obsolete.

Lilopristone (INN) is a synthetic, steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and was patented in 1985. It is described as an abortifacient and endometrial contraceptive. The drug differs from mifepristone only in the structure of its C17α side chain, and is said to have much reduced antiglucocorticoid activity in comparison.

Oxprenoate potassium is a synthetic steroidal antimineralocorticoid which was never marketed. The affinities of oxprenoate potassium for the steroid hormone receptors have been reported.

Pirenperone (INN, USAN, BAN; developmental code names R-47456, R-50656) is a serotonin receptor antagonist described as an antipsychotic and tranquilizer which was never marketed. It is a relatively selective antagonist of the serotonin 5-HT2 receptors and has been used in scientific research to study the serotonin system. In the 1980s, the drug was found to block the effects of the lysergic acid diethylamide (LSD) in animals, and along with ketanserin, led to the elucidation of the 5-HT2A receptor as the biological mediator of the effects of serotonergic psychedelics.