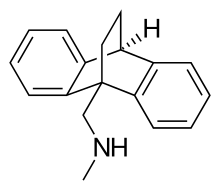
An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Benzodiazepines, colloquially called "benzos", are a class of depressant drugs whose core chemical structure is the fusion of a benzene ring and a diazepine ring. They are prescribed to treat conditions such as anxiety disorders, insomnia, and seizures. The first benzodiazepine, chlordiazepoxide (Librium), was discovered accidentally by Leo Sternbach in 1955, and was made available in 1960 by Hoffmann–La Roche, which followed with the development of diazepam (Valium) three years later, in 1963. By 1977, benzodiazepines were the most prescribed medications globally; the introduction of selective serotonin reuptake inhibitors (SSRIs), among other factors, decreased rates of prescription, but they remain frequently used worldwide.

Diazepam, sold under the brand name Valium among others, is a medicine of the benzodiazepine family that acts as an anxiolytic. It is used to treat a range of conditions, including anxiety, seizures, alcohol withdrawal syndrome, muscle spasms, insomnia, and restless legs syndrome. It may also be used to cause memory loss during certain medical procedures. It can be taken orally, as a suppository inserted into the rectum, intramuscularly, intravenously or used as a nasal spray. When injected intravenously, effects begin in one to five minutes and last up to an hour. When taken by mouth, effects begin after 15 to 60 minutes.
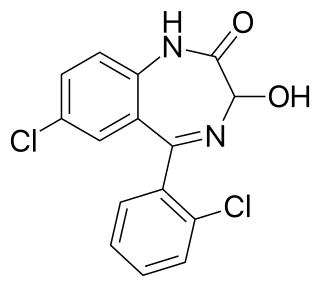
Lorazepam, sold under the brand name Ativan among others, is a benzodiazepine medication. It is used to treat anxiety, trouble sleeping, severe agitation, active seizures including status epilepticus, alcohol withdrawal, and chemotherapy-induced nausea and vomiting. It is also used during surgery to interfere with memory formation and to sedate those who are being mechanically ventilated. It is also used, along with other treatments, for acute coronary syndrome due to cocaine use. It can be given orally, transdermal, intravenously (IV), or intramuscularly When given by injection, onset of effects is between one and thirty minutes and effects last for up to a day.

Oxazepam is a short-to-intermediate-acting benzodiazepine. Oxazepam is used for the treatment of anxiety and insomnia and in the control of symptoms of alcohol withdrawal syndrome.
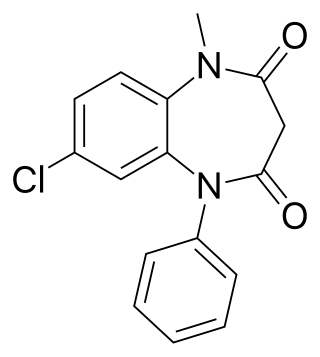
Clobazam, sold under the brand names Frisium, Onfi and others, is a benzodiazepine class medication that was patented in 1968. Clobazam was first synthesized in 1966 and first published in 1969. Clobazam was originally marketed as an anxioselective anxiolytic since 1970, and an anticonvulsant since 1984. The primary drug-development goal was to provide greater anxiolytic, anti-obsessive efficacy with fewer benzodiazepine-related side effects.

Nordazepam is a 1,4-benzodiazepine derivative. Like other benzodiazepine derivatives, it has amnesic, anticonvulsant, anxiolytic, muscle relaxant, and sedative properties. However, it is used primarily in the treatment of anxiety disorders. It is an active metabolite of diazepam, chlordiazepoxide, clorazepate, prazepam, pinazepam, and medazepam.

Clorazepate, sold under the brand name Tranxene among others, is a benzodiazepine medication. It possesses anxiolytic, anticonvulsant, sedative, hypnotic, and skeletal muscle relaxant properties. Clorazepate is an unusually long-lasting benzodiazepine and serves as a prodrug for the equally long-lasting desmethyldiazepam, which is rapidly produced as an active metabolite. Desmethyldiazepam is responsible for most of the therapeutic effects of clorazepate.

Alpidem, sold under the brand name Ananxyl, is a nonbenzodiazepine anxiolytic medication which was briefly used to treat anxiety disorders but is no longer marketed. It was previously marketed in France, but was discontinued due to liver toxicity. Alpidem is taken by mouth.

Camazepam is a benzodiazepine psychoactive drug, marketed under the brand names Albego, Limpidon and Paxor. It is the dimethyl carbamate ester of temazepam, a metabolite of diazepam. While it possesses anxiolytic, anticonvulsant, skeletal muscle relaxant and hypnotic properties it differs from other benzodiazepines in that its anxiolytic properties are particularly prominent but has comparatively limited anticonvulsant, hypnotic and skeletal muscle relaxant properties.

Etizolam is a thienodiazepine derivative which is a benzodiazepine analog. The etizolam molecule differs from a benzodiazepine in that the benzene ring has been replaced by a thiophene ring and triazole ring has been fused, making the drug a thienotriazolodiazepine.
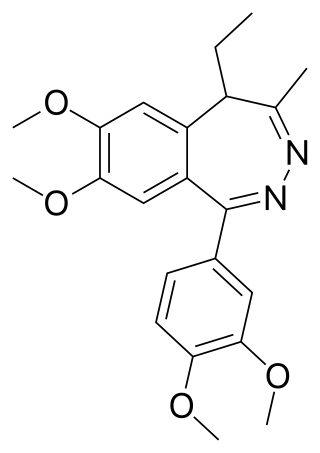
Tofisopam is an anxiolytic that is marketed in several European countries. Chemically, it is a 2,3-benzodiazepine. Unlike other anxiolytic benzodiazepines however, tofisopam does not have anticonvulsant, sedative, skeletal muscle relaxant, motor skill-impairing or amnestic properties. While it may not be an anticonvulsant in and of itself, it has been shown to enhance the anticonvulsant action of classical 1,4-benzodiazepines and muscimol, but not sodium valproate, carbamazepine, phenobarbital, or phenytoin. Tofisopam is indicated for the treatment of anxiety and alcohol withdrawal, and is prescribed in a dosage of 50–300 mg per day divided into three doses. Peak plasma levels are attained two hours after an oral dose. Tofisopam is not reported as causing dependence to the same extent as other benzodiazepines, but is still recommended to be prescribed for a maximum of 12 weeks.

Bretazenil (Ro16-6028) is an imidazopyrrolobenzodiazepine anxiolytic drug which is derived from the benzodiazepine family, and was invented in 1988. It is most closely related in structure to the GABA antagonist flumazenil, although its effects are somewhat different. It is classified as a high-potency benzodiazepine due to its high affinity binding to benzodiazepine binding sites where it acts as a partial agonist. Its profile as a partial agonist and preclinical trial data suggests that it may have a reduced adverse effect profile. In particular bretazenil has been proposed to cause a less strong development of tolerance and withdrawal syndrome. Bretazenil differs from traditional 1,4-benzodiazepines by being a partial agonist and because it binds to α1, α2, α3, α4, α5 and α6 subunit containing GABAA receptor benzodiazepine receptor complexes. 1,4-benzodiazepines bind only to α1, α2, α3 and α5GABAA benzodiazepine receptor complexes.

Chlordiazepoxide, trade name Librium among others, is a sedative and hypnotic medication of the benzodiazepine class; it is used to treat anxiety, insomnia and symptoms of withdrawal from alcohol and other drugs.

Delorazepam, also known as chlordesmethyldiazepam and nordiclazepam, is a drug which is a benzodiazepine and a derivative of desmethyldiazepam. It is marketed in Italy, where it is available under the trade name EN and Dadumir. Delorazepam (chlordesmethyldiazepam) is also an active metabolite of the benzodiazepine drugs diclazepam and cloxazolam. Adverse effects may include hangover type effects, drowsiness, behavioural impairments and short-term memory impairments. Similar to other benzodiazepines delorazepam has anxiolytic, skeletal muscle relaxant, hypnotic and anticonvulsant properties.

Alcohol detoxification is the abrupt cessation of alcohol intake in individuals that have alcohol use disorder. This process is often coupled with substitution of drugs that have effects similar to the effects of alcohol in order to lessen the symptoms of alcohol withdrawal. When withdrawal does occur, it results in symptoms of varying severity.

Fosazepam is a drug which is a benzodiazepine derivative; it is a water soluble derivative of diazepam. It has sedative and anxiolytic effects, and is a derivative of diazepam which has been substituted with a dimethylphosphoryl group to improve solubility in water.

Metaclazepam is a drug which is a benzodiazepine derivative. It is a relatively selective anxiolytic with less sedative or muscle relaxant properties than other benzodiazepines such as diazepam or bromazepam. It has an active metabolite N-desmethylmetaclazepam, which is the main metabolite of metaclazepam. There is no significant difference in metabolism between younger and older individuals.

Premazepam is a Pyrrolodiazepine class of drug. It is a partial agonist of benzodiazepine receptors and was shown in 1984 to possess both anxiolytic and sedative properties in humans but was never marketed.
Deramciclane (EGIS-3886) is a non-benzodiazepine-type anxiolytic drug to treat various types of anxiety disorders. Deramciclane is a unique alternative to current anxiolytics on the market because it has a novel chemical structure and target. It acts as an antagonist at the 5-HT2A receptor, as an inverse agonist at the 5-HT2C receptor, and as a GABA reuptake inhibitor. The two serotonin receptors are G protein-coupled receptors and are two of the main excitatory serotonin receptor types. Their excitation has been implicated in anxiety and mood. Deramciclane does not affect CYP3A4 activity in metabolizing other drugs, but it is a weak inhibitor of CYP2D6. Some studies also show the drug to have moderate affinity to dopamine D2 receptors and low affinity to dopamine receptor D1. Researchers are looking for alternatives to benzodiazepines for anxiolytic use because benzodiazepine drugs have sedative and muscle relaxant side effects.