Methicillin-resistant Staphylococcus aureus (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of Staphylococcus aureus. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019.

Linezolid is an antibiotic used for the treatment of infections caused by Gram-positive bacteria that are resistant to other antibiotics. Linezolid is active against most Gram-positive bacteria that cause disease, including streptococci, vancomycin-resistant enterococci (VRE), and methicillin-resistant Staphylococcus aureus (MRSA). The main uses are infections of the skin and pneumonia although it may be used for a variety of other infections including drug-resistant tuberculosis. It is used either by injection into a vein or by mouth.

Clindamycin is an antibiotic medication used for the treatment of a number of bacterial infections, including osteomyelitis (bone) or joint infections, pelvic inflammatory disease, strep throat, pneumonia, acute otitis media, and endocarditis. It can also be used to treat acne, and some cases of methicillin-resistant Staphylococcus aureus (MRSA). In combination with quinine, it can be used to treat malaria. It is available by mouth, by injection into a vein, and as a cream or a gel to be applied to the skin or in the vagina.

Tigecycline, sold under the brand name Tygacil, is a tetracycline antibiotic medication for a number of bacterial infections. It is a glycylcycline administered intravenously. It was developed in response to the growing rate of antibiotic resistant bacteria such as Staphylococcus aureus, Acinetobacter baumannii, and E. coli. As a tetracycline derivative antibiotic, its structural modifications has expanded its therapeutic activity to include Gram-positive and Gram-negative organisms, including those of multi-drug resistance.
2-Oxazolidone is a heterocyclic organic compound containing both nitrogen and oxygen in a 5-membered ring.
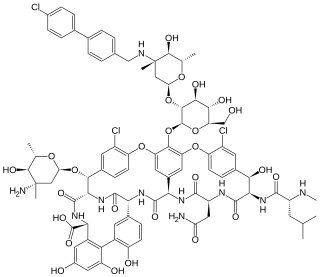
Oritavancin, sold under the brand name Orbactiv among others, is a semisynthetic glycopeptide antibiotic medication for the treatment of serious Gram-positive bacterial infections. Its chemical structure as a lipoglycopeptide is similar to vancomycin.

Dalbavancin, sold under the brand names Dalvance in the US and Xydalba in the EU among others, is a second-generation lipoglycopeptide antibiotic medication. It belongs to the same class as vancomycin, the most widely used and one of the treatments available to people infected with methicillin-resistant Staphylococcus aureus (MRSA).
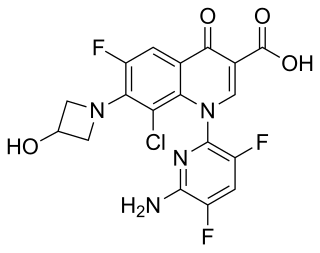
Delafloxacin sold under the brand name Baxdela among others, is a fluoroquinolone antibiotic used to treat acute bacterial skin and skin structure infections.

Ceftaroline fosamil (INN), brand name Teflaro in the US and Zinforo in Europe, is a cephalosporin antibiotic with anti-MRSA activity. Ceftaroline fosamil is a prodrug of ceftaroline. It is active against methicillin-resistant Staphylococcus aureus (MRSA) and other Gram-positive bacteria. It retains some activity of later-generation cephalosporins having broad-spectrum activity against Gram-negative bacteria, but its effectiveness is relatively much weaker. It is currently being investigated for community-acquired pneumonia and complicated skin and skin structure infection.
Skin and skin structure infections (SSSIs), also referred to as skin and soft tissue infections (SSTIs), or acute bacterial skin and skin structure infections (ABSSSIs), are infections of skin and associated soft tissues. Historically, the pathogen involved has most frequently been a bacterial species—always, since redescription of SSSIs as ABSSSIs—and as such, these infections require treatment by antibiotics.
Linopristin/flopristin is an experimental drug candidate under development by Novexel. It is an oral streptogramin antibiotic that has potent in vitro activity against certain Gram-positive bacteria including methicillin resistant Staphylococcus aureus (MRSA), as well as the important respiratory pathogens including penicillin-, macrolide- and quinolone-resistant strains. It is a combination of linopristin and flopristin.

Lipoglycopeptides are a class of antibiotic that have lipophilic side-chains linked to glycopeptides. The class includes oritavancin, telavancin and dalbavancin.
Taksta is a front-loaded oral dosing regimen of sodium fusidate under development in the U.S. as an antibiotic for gram-positive infections including drug-resistant strains such as methicillin-resistant Staphylococcus aureus.

Sophoraflavanone G is a volatile phytoncide, released into the atmosphere, soil and ground water, by members of the Sophora genus. Due to an increase in the rates of antibiotic-resistant bacteria, scientific efforts have focused on finding either naturally-made or genetically modified compounds that can treat and or prevent these harmful and sometimes deadly bacteria. Sophoraflavanone G, due to its use as a phytoncide, has been found to impact the growth of antibiotic-resistant bacteria and enhance the effect of currently used antibiotics.

Cubist Pharmaceuticals was an American biopharmaceutical company that targeted pathogens like MRSA.
Trius Therapeutics was a biopharma company based in San Diego, CA that focused on the development of antibiotics.

Omadacycline, sold under the brand name Nuzyra, is a broad spectrum antibiotic medication belonging to the aminomethylcycline subclass of tetracycline antibiotics. In the United States, it was approved in October 2018, for the treatment of community-acquired bacterial pneumonia and acute skin and skin structure infections.

Lefamulin, sold under the brand name Xenleta, is an antibiotic medication used it to treat adults with community-acquired bacterial pneumonia. It is taken by mouth or by injection into a vein.

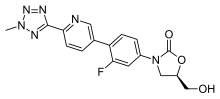
Contezolid is an antibiotic of the oxazolidinone class. It is effective against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pyogenes, Streptococcus agalactiae, and other bacteria.