5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.

Imipramine, sold under the brand name Tofranil, among others, is a tricyclic antidepressant (TCA) mainly used in the treatment of depression. It is also effective in treating anxiety and panic disorder. Imipramine is taken by mouth.

Desipramine, sold under the brand name Norpramin among others, is a tricyclic antidepressant (TCA) used in the treatment of depression. It acts as a relatively selective norepinephrine reuptake inhibitor, though it does also have other activities such as weak serotonin reuptake inhibitory, α1-blocking, antihistamine, and anticholinergic effects. The drug is not considered a first-line treatment for depression since the introduction of selective serotonin reuptake inhibitor (SSRI) antidepressants, which have fewer side effects and are safer in overdose.

Mianserin, sold under the brand name Tolvon among others, is an atypical antidepressant that is used primarily in the treatment of depression in Europe and elsewhere in the world. It is a tetracyclic antidepressant (TeCA). Mianserin is closely related to mirtazapine, both chemically and in terms of its actions and effects, although there are significant differences between the two drugs.
Nicergoline, sold under the brand name Sermion among others, is an ergot derivative used to treat senile dementia and other disorders with vascular origins. Internationally it has been used for frontotemporal dementia as well as early onset in Lewy body dementia and Parkinson's dementia. It decreases vascular resistance and increases arterial blood flow in the brain, improving the utilization of oxygen and glucose by brain cells. It has similar vasoactive properties in other areas of the body, particularly the lungs. Unlike many other ergolines, such as ergotamine, nicergoline is not associated with cardiac fibrosis.
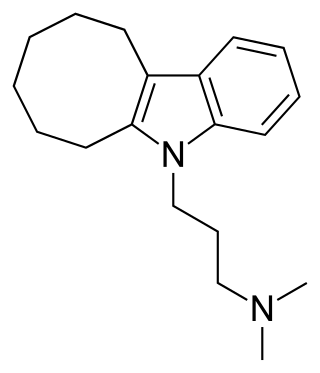
Iprindole, sold under the brand names Prondol, Galatur, and Tertran, is an atypical tricyclic antidepressant (TCA) that has been used in the United Kingdom and Ireland for the treatment of depression but appears to no longer be marketed. It was developed by Wyeth and was marketed in 1967. The drug has been described by some as the first "second-generation" antidepressant to be introduced. However, it was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

RS-67,333 is a drug which has been investigated as a potential rapid-acting antidepressant, nootropic, and treatment for Alzheimer's disease. It is a high-affinity 5-HT4 receptor partial agonist, as well as a sigma receptor ligand of both subtypes to a lesser extent.

5-Hydroxytryptamine receptor 4 is a protein that in humans is encoded by the HTR4 gene.

The serotonin 1A receptor is a subtype of serotonin receptors, or 5-HT receptors, that binds serotonin, also known as 5-HT, a neurotransmitter. 5-HT1A is expressed in the brain, spleen, and neonatal kidney. It is a G protein-coupled receptor (GPCR), coupled to the Gi protein, and its activation in the brain mediates hyperpolarisation and reduction of firing rate of the postsynaptic neuron. In humans, the serotonin 1A receptor is encoded by the HTR1A gene.

Sarpogrelate is a drug which acts as an antagonist at the 5HT2A and 5-HT2B receptors. It blocks serotonin-induced platelet aggregation, and has applications in the treatment of many diseases including diabetes mellitus, Buerger's disease, Raynaud's disease, coronary artery disease, angina pectoris, and atherosclerosis.
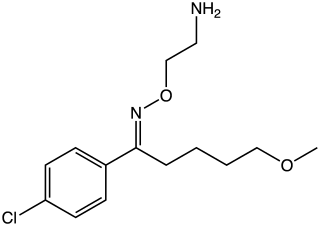
Clovoxamine (INN) is a drug that was discovered in the 1970s and was subsequently investigated as an antidepressant and anxiolytic agent but was never marketed. It acts as a serotonin-norepinephrine reuptake inhibitor (SNRI), with little affinity for the muscarinic acetylcholine, histamine, adrenergic, and serotonin receptors. The compound is structurally related to fluvoxamine.

Oxaprotiline, also known as hydroxymaprotiline, is a norepinephrine reuptake inhibitor belonging to the tetracyclic antidepressant (TeCA) family and is related to maprotiline. Though investigated as an antidepressant, it was never marketed.

Bifemelane (INN) (Alnert, Celeport), or bifemelane hydrochloride (JAN), also known as 4-(O-benzylphenoxy)-N-methylbutylamine, is an antidepressant and cerebral activator that is widely used in the treatment of cerebral infarction patients with depressive symptoms in Japan, and in the treatment of senile dementia as well. It also appears to be useful in the treatment of glaucoma. Bifemelane acts as a monoamine oxidase inhibitor (MAOI) of both isoenzymes, with competitive (reversible) inhibition of MAO-A (Ki = 4.20 μM) (making it a reversible inhibitor of monoamine oxidase A (RIMA)) and non-competitive (irreversible) inhibition of MAO-B (Ki = 46.0 μM), and also acts (weakly) as a norepinephrine reuptake inhibitor. The drug has nootropic, neuroprotective, and antidepressant-like effects in animal models, and appears to enhance the cholinergic system in the brain.
A serotonin releasing agent (SRA) is a type of drug that induces the release of serotonin into the neuronal synaptic cleft. A selective serotonin releasing agent (SSRA) is an SRA with less significant or no efficacy in producing neurotransmitter efflux at other types of monoamine neurons.

RDS-127 is a drug which is used in scientific research. It acts as a D2-like receptor agonist and also has some serotonin and adrenergic agonist effects, as well as some anticholinergic action, and produces both anorectic and pro-sexual effects in animal studies.

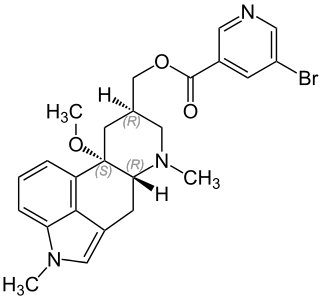
Capeserod (INN; development code SL65.0155) is a selective 5-HT4 receptor partial agonist with Ki = 0.6 nM and IA = 40–50% (relative to serotonin). It potently enhances cognition, learning, and memory, and also possesses antidepressant effects. Capeserod was in phase II clinical trials around 2004–2006 for the treatment of memory deficits and dementia but no new information has surfaced since and it appears to have been abandoned.

GR-113808 is a drug which acts as a potent and selective 5-HT4 serotonin receptor antagonist. It is used in researching the roles of 5-HT4 receptors in various processes, and has been used to test some of the proposed therapeutic effects of selective 5-HT4 agonists, such as for instance blocking the nootropic effects of 5-HT4 agonists, and worsening the respiratory depression produced by opioid analgesic drugs, which appears to be partly 5-HT4 mediated and can be counteracted by certain 5-HT4 agonists.

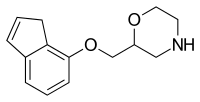
Teniloxazine, also known as sufoxazine and sulfoxazine, is a drug which is marketed in Japan. Though initially investigated as a neuroprotective and nootropic agent for the treatment of cerebrovascular insufficiency in the 1980s, it was ultimately developed and approved as an antidepressant instead. It acts as a potent norepinephrine reuptake inhibitor, with fair selectivity over the serotonin and dopamine transporters, and also behaves as an antagonist of the 5-HT2A receptor.
A cerebral activator, or cerebral metabolic enhancer, is a type of drug that "activates" the central nervous system in the context of cerebrovascular diseases such as stroke and dementia. The term has been used specifically to describe a few Japanese drugs, such as indeloxazine and bifemelane.