
Substance P (SP) is an undecapeptide and a type of neuropeptide, belonging to the tachykinin family of neuropeptides. It acts as a neurotransmitter and a neuromodulator. Substance P and the closely related neurokinin A (NKA) are produced from a polyprotein precursor after alternative splicing of the preprotachykinin A gene. The deduced amino acid sequence of substance P is as follows:

In excitotoxicity, nerve cells suffer damage or death when the levels of otherwise necessary and safe neurotransmitters such as glutamate become pathologically high, resulting in excessive stimulation of receptors. For example, when glutamate receptors such as the NMDA receptor or AMPA receptor encounter excessive levels of the excitatory neurotransmitter, glutamate, significant neuronal damage might ensue. Excess glutamate allows high levels of calcium ions (Ca2+) to enter the cell. Ca2+ influx into cells activates a number of enzymes, including phospholipases, endonucleases, and proteases such as calpain. These enzymes go on to damage cell structures such as components of the cytoskeleton, membrane, and DNA. In evolved, complex adaptive systems such as biological life it must be understood that mechanisms are rarely, if ever, simplistically direct. For example, NMDA in subtoxic amounts induces neuronal survival of otherwise toxic levels of glutamate.

Dizocilpine (INN), also known as MK-801, is a pore blocker of the NMDA receptor, a glutamate receptor, discovered by a team at Merck in 1982. Glutamate is the brain's primary excitatory neurotransmitter. The channel is normally blocked with a magnesium ion and requires depolarization of the neuron to remove the magnesium and allow the glutamate to open the channel, causing an influx of calcium, which then leads to subsequent depolarization. Dizocilpine binds inside the ion channel of the receptor at several of PCP's binding sites thus preventing the flow of ions, including calcium (Ca2+), through the channel. Dizocilpine blocks NMDA receptors in a use- and voltage-dependent manner, since the channel must open for the drug to bind inside it. The drug acts as a potent anti-convulsant and probably has dissociative anesthetic properties, but it is not used clinically for this purpose because of the discovery of brain lesions, called Olney's lesions (see below), in laboratory rats. Dizocilpine is also associated with a number of negative side effects, including cognitive disruption and psychotic-spectrum reactions. It inhibits the induction of long term potentiation and has been found to impair the acquisition of difficult, but not easy, learning tasks in rats and primates. Because of these effects of dizocilpine, the NMDA receptor pore blocker ketamine is used instead as a dissociative anesthetic in human medical procedures. While ketamine may also trigger temporary psychosis in certain individuals, its short half-life and lower potency make it a much safer clinical option. However, dizocilpine is the most frequently used uncompetitive NMDA receptor antagonist in animal models to mimic psychosis for experimental purposes.

Neuroprotection refers to the relative preservation of neuronal structure and/or function. In the case of an ongoing insult the relative preservation of neuronal integrity implies a reduction in the rate of neuronal loss over time, which can be expressed as a differential equation.

The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.

Huperzine A is a naturally-occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a 2013 meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine (ACh) by the enzyme acetylcholinesterase. It is also an antagonist of the NMDA-receptor. It is commonly available over the counter as a nutritional supplement and marketed as a memory and concentration enhancer.
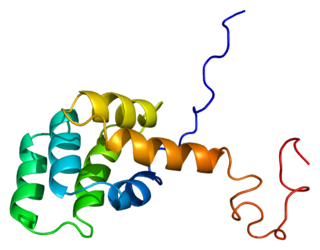
The Fas receptor, also known as Fas, FasR, apoptosis antigen 1, cluster of differentiation 95 (CD95) or tumor necrosis factor receptor superfamily member 6 (TNFRSF6), is a protein that in humans is encoded by the FAS gene. Fas was first identified using a monoclonal antibody generated by immunizing mice with the FS-7 cell line. Thus, the name Fas is derived from FS-7-associated surface antigen.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for humans and animals; the state of anesthesia they induce is referred to as dissociative anesthesia.

Presenilin-1(PS-1) is a presenilin protein that in humans is encoded by the PSEN1 gene. Presenilin-1 is one of the four core proteins in the gamma secretase complex, which is considered to play an important role in generation of amyloid beta (Aβ) from amyloid-beta precursor protein (APP). Accumulation of amyloid beta is associated with the onset of Alzheimer's disease.

Tumor necrosis factor receptor 1 (TNFR1), also known as tumor necrosis factor receptor superfamily member 1A (TNFRSF1A) and CD120a, is a ubiquitous membrane receptor that binds tumor necrosis factor-alpha (TNFα).

Prostaglandin E2 receptor 4 (EP4) is a prostaglandin receptor for prostaglandin E2 (PGE2) encoded by the PTGER4 gene in humans; it is one of four identified EP receptors, the others being EP1, EP2, and EP3, all of which bind with and mediate cellular responses to PGE2 and also, but generally with lesser affinity and responsiveness, certain other prostanoids (see Prostaglandin receptors). EP4 has been implicated in various physiological and pathological responses in animal models and humans.

Glutamate [NMDA] receptor subunit epsilon-2, also known as N-methyl D-aspartate receptor subtype 2B, is a protein that in humans is encoded by the GRIN2B gene.

Free fatty acid receptor 1 (FFAR1), also known as G-protein coupled receptor 40 (GPR40), is a rhodopsin-like G-protein coupled receptor that is coded by the FFAR1 gene. This gene is located on the short arm of chromosome 19 at position 13.12. G protein-coupled receptors reside on their parent cells' surface membranes, bind any one of the specific set of ligands that they recognize, and thereby are activated to trigger certain responses in their parent cells. FFAR1 is a member of a small family of structurally and functionally related GPRs termed free fatty acid receptors (FFARs). This family includes at least three other FFARs viz., FFAR2, FFAR3, and FFAR4. FFARs bind and thereby are activated by certain fatty acids.

The 5HT6 receptor is a subtype of 5HT receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5HT). It is a G protein-coupled receptor (GPCR) that is coupled to Gs and mediates excitatory neurotransmission. HTR6 denotes the human gene encoding for the receptor.
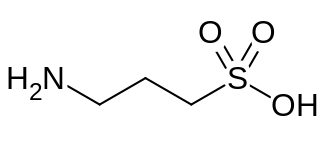
Homotaurine is a natural sulfonic acid found in seaweed. It is analogous to taurine, but with an extra carbon in its chain. It has GABAergic activity, apparently by mimicking GABA, which it resembles.

Alzheimer's disease (AD) is a neurodegenerative disease that usually starts slowly and progressively worsens, and is the cause of 60–70% of cases of dementia. The most common early symptom is difficulty in remembering recent events. As the disease advances, symptoms can include problems with language, disorientation, mood swings, loss of motivation, self-neglect, and behavioral issues. As a person's condition declines, they often withdraw from family and society. Gradually, bodily functions are lost, ultimately leading to death. Although the speed of progression can vary, the average life expectancy following diagnosis is three to twelve years.
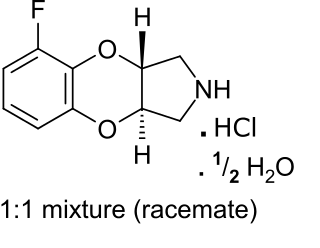
Fluparoxan is a potent α2-adrenergic receptor antagonist with excellent selectivity for this receptor over the α1-adrenergic receptor (2,630-fold), and is the only well-studied α2-adrenergic receptor antagonist in its structural family which does not antagonize any variant of the imidazoline receptor. It was shown to possess central α2-adrenoceptor antagonist activity after oral doses in man and was patented as an antidepressant by Glaxo in the early 1980s, but its development was discontinued when the compound failed to show a clear clinical advantage over existing therapies.
Conantokins are a small family of helical peptides that are derived from the venom of predatory marine snails of the genus Conus. Conantokins act as potent and specific antagonists of the N-methyl-D-aspartate receptor (NMDAR). They are the only naturally-derived peptides to do so. The subtypes of conantokins exhibit a surprising variability of selectivity across the NMDAR subunits, and are therefore uniquely useful in developing subunit-specific pharmacological probes.
In molecular biology mir-153 microRNA is a short RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms.
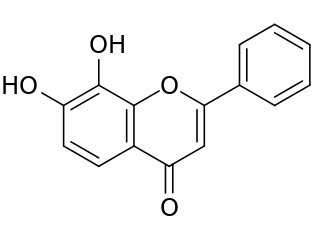
Tropoflavin, also known as 7,8-dihydroxyflavone, is a naturally occurring flavone found in Godmania aesculifolia, Tridax procumbens, and primula tree leaves. It has been found to act as a potent and selective small-molecule agonist of the tropomyosin receptor kinase B (TrkB), the main signaling receptor of the neurotrophin brain-derived neurotrophic factor (BDNF). Tropoflavin is both orally bioavailable and able to penetrate the blood–brain barrier. A prodrug of tropoflavin with greatly improved potency and pharmacokinetics, R13, is under development for the treatment of Alzheimer's disease.


















