
N-methyl-D-aspartic acid or N-methyl-D-aspartate (NMDA) is an amino acid derivative that acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter which normally acts at that receptor. Unlike glutamate, NMDA only binds to and regulates the NMDA receptor and has no effect on other glutamate receptors. NMDA receptors are particularly important when they become overactive during, for example, withdrawal from alcohol as this causes symptoms such as agitation and, sometimes, epileptiform seizures.

The α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor is an ionotropic transmembrane receptor for glutamate (iGluR) that mediates fast synaptic transmission in the central nervous system (CNS). It has been traditionally classified as a non-NMDA-type receptor, along with the kainate receptor. Its name is derived from its ability to be activated by the artificial glutamate analog AMPA. The receptor was first named the "quisqualate receptor" by Watkins and colleagues after a naturally occurring agonist quisqualate and was only later given the label "AMPA receptor" after the selective agonist developed by Tage Honore and colleagues at the Royal Danish School of Pharmacy in Copenhagen. The GRIA2-encoded AMPA receptor ligand binding core was the first glutamate receptor ion channel domain to be crystallized.

The N-methyl-D-aspartatereceptor (also known as the NMDA receptor or NMDAR), is a glutamate receptor and ion channel found in neurons. The NMDA receptor is one of three types of ionotropic glutamate receptors, the other two being AMPA and kainate receptors. Depending on its subunit composition, its ligands are glutamate and glycine (or D-serine). However, the binding of the ligands is typically not sufficient to open the channel as it may be blocked by Mg2+ ions which are only removed when the neuron is sufficiently depolarized. Thus, the channel acts as a “coincidence detector” and only once both of these conditions are met, the channel opens and it allows positively charged ions (cations) to flow through the cell membrane. The NMDA receptor is thought to be very important for controlling synaptic plasticity and mediating learning and memory functions.

CNQX or cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) is a competitive AMPA/kainate receptor antagonist. Its chemical formula is C9H4N4O4. CNQX is often used in the retina to block the responses of OFF-bipolar cells for electrophysiology recordings.
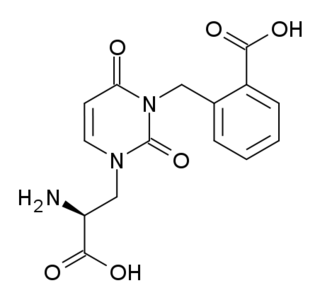
UBP-302 is a highly selective kainate receptor antagonist used in the study of many neurological processes. It is active at micromolar concentration within an in vitro preparation and specifically targets the GluK1 (iGluR5) subunit of the receptor. This compound was developed at the University of Bristol.

Kainate receptors, or kainic acid receptors (KARs), are ionotropic receptors that respond to the neurotransmitter glutamate. They were first identified as a distinct receptor type through their selective activation by the agonist kainate, a drug first isolated from the algae Digenea simplex. They have been traditionally classified as a non-NMDA-type receptor, along with the AMPA receptor. KARs are less understood than AMPA and NMDA receptors, the other ionotropic glutamate receptors. Postsynaptic kainate receptors are involved in excitatory neurotransmission. Presynaptic kainate receptors have been implicated in inhibitory neurotransmission by modulating release of the inhibitory neurotransmitter GABA through a presynaptic mechanism.

The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.
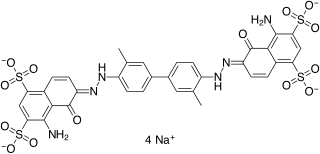
T-1824 or Evans blue, often incorrectly rendered as Evan's blue, is an azo dye that has a very high affinity for serum albumin. Because of this, it can be useful in physiology in estimating the proportion of body water contained in blood plasma. It fluoresces with excitation peaks at 470 and 540 nm and an emission peak at 680 nm.

Quisqualic acid is an agonist of the AMPA, kainate, and group I metabotropic glutamate receptors. It is one of the most potent AMPA receptor agonists known. It causes excitotoxicity and is used in neuroscience to selectively destroy neurons in the brain or spinal cord. Quisqualic acid occurs naturally in the seeds of Quisqualis species.

The muscarinic acetylcholine receptor M1, also known as the cholinergic receptor, muscarinic 1, is a muscarinic receptor that in humans is encoded by the CHRM1 gene. It is localized to 11q13.

Metabotropic glutamate receptor 4 is a protein that in humans is encoded by the GRM4 gene.
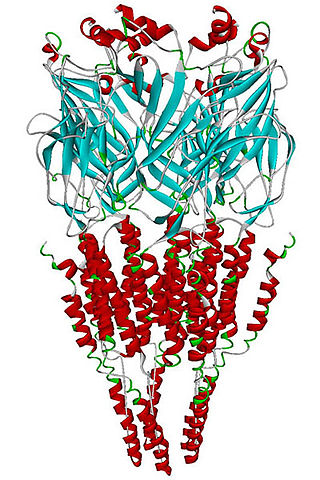
The alpha-7 nicotinic receptor, also known as the α7 receptor, is a type of nicotinic acetylcholine receptor implicated in long-term memory, consisting entirely of α7 subunits. As with other nicotinic acetylcholine receptors, functional α7 receptors are pentameric [i.e., (α7)5 stoichiometry].

IDRA-21 is a positive allosteric modulator of the AMPA receptor and a benzothiadiazine derivative. It is a chiral molecule, with (+)-IDRA-21 being the active form.

Cyclothiazide, sometimes abbreviated CTZ, is a benzothiadiazide (thiazide) diuretic and antihypertensive that was originally introduced in the United States in 1963 by Eli Lilly and was subsequently also marketed in Europe and Japan. Related drugs include diazoxide, hydrochlorothiazide, and chlorothiazide.

5-Iodowillardiine is a selective agonist for the kainate receptor, with only limited effects at the AMPA receptor. It is selective for kainate receptors composed of GluR5 subunits. It is an excitotoxic neurotoxin in vivo, but has proved highly useful for characterising the subtypes and function of the various kainate receptors in the brain and spinal cord.
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimuli. Some of them, like benzodiazepines or alcoholic beverages, function as psychoactive drugs. The site that an allosteric modulator binds to is not the same one to which an endogenous agonist of the receptor would bind. Modulators and agonists can both be called receptor ligands.
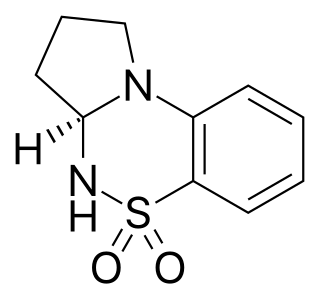
S-18986 is a positive allosteric modulator of the AMPA receptor related to cyclothiazide. It has nootropic and neuroprotective effects in animal studies, and induces both production of BDNF and AMPA-mediated release of noradrenaline and acetylcholine in the hippocampus and frontal cortex of the brain.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

Willardiine (correctly spelled with two successive i's) or (S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione is a chemical compound that occurs naturally in the seeds of Mariosousa willardiana and Acacia sensu lato. The seedlings of these plants contain enzymes capable of complex chemical substitutions that result in the formation of free amino acids (See: #Synthesis). Willardiine is frequently studied for its function in higher level plants. Additionally, many derivates of willardiine are researched for their potential in pharmaceutical development. Willardiine was first discovered in 1959 by R. Gmelin, when he isolated several free, non-protein amino acids from Acacia willardiana (another name for Mariosousa willardiana) when he was studying how these families of plants synthesize uracilyalanines. A related compound, Isowillardiine, was concurrently isolated by a different group, and it was discovered that the two compounds had different structural and functional properties. Subsequent research on willardiine has focused on the functional significance of different substitutions at the nitrogen group and the development of analogs of willardiine with different pharmacokinetic properties. In general, Willardiine is the one of the first compounds studied in which slight changes to molecular structure result in compounds with significantly different pharmacokinetic properties.
TAK-653 is an experimental drug being investigated as a treatment for treatment-resistant depression. It is being developed by Takeda.




















