
The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.

NMDA receptor antagonists are a class of drugs that work to antagonize, or inhibit the action of, the N-Methyl-D-aspartate receptor (NMDAR). They are commonly used as anesthetics for animals and humans; the state of anesthesia they induce is referred to as dissociative anesthesia.
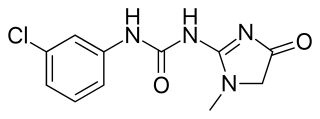
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 5 is an excitatory Gq-coupled G protein-coupled receptor predominantly expressed on the postsynaptic sites of neurons. In humans, it is encoded by the GRM5 gene.

Metabotropic glutamate receptor 7 is a protein that in humans is encoded by the GRM7 gene.

Eglumetad is a research drug developed by Eli Lilly and Company, which is being investigated for its potential in the treatment of anxiety and drug addiction. It is a glutamate derived compound and its mode of action implies a novel mechanism.
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

Tezampanel is a drug originally developed by Eli Lilly which acts as a competitive antagonist of the AMPA and kainate subtypes of the ionotropic glutamate receptor family, with selectivity for the GluR5 subtype of the kainate receptor. It has neuroprotective and anticonvulsant properties, the former of which may, at least in part, occur via blockade of calcium uptake into neurons.

AMN082 is a selective metabotropic glutamate receptor 7 (mGluR7) allosteric agonist. It mimics the effect of glutamate. AMN082 is the first selective mGluR7 agonist and has expanded the potential array of research opportunities on the effects of mGluR7 in the central nervous system.

3-( ethynyl)pyridine (MTEP) is a research drug that was developed by Merck & Co. as a selective allosteric antagonist of the metabotropic glutamate receptor subtype mGluR5. Identified through structure-activity relationship studies on an older mGluR5 antagonist MPEP, MTEP has subsequently itself acted as a lead compound for newer and even more improved drugs.

EGLU is a drug that is used in neuroscience research. It was one of the first compounds found that acts as a selective antagonist for the group II metabotropic glutamate receptors (mGluR2/3), and so has been useful in the characterization and study of this receptor subfamily.

PHCCC is a research drug which acts as a glutamate receptor ligand, particularly being a positive allosteric modulator at the mGluR4 subtype, as well as an agonist at mGluR6. It has anxiolytic effects in animal studies. PHCCC and similar drugs have been suggested as novel treatments for Parkinson's disease.

SIB-1757 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anti-hyperalgesia effects in animals. SIB-1757 along with other mGluR5 antagonists has been shown to have neuroprotective and hepatoprotective effects, and it is also used to study the role of the mGluR5 receptor in brain development.

SIB-1893 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anticonvulsant and neuroprotective effects, and reduces glutamate release. It has also been found to act as a positive allosteric modulator of mGluR4.

CDPPB is a drug used in scientific research which acts as a positive allosteric modulator selective for the metabotropic glutamate receptor subtype mGluR5. It has antipsychotic effects in animal models, and mGluR5 modulators are under investigation as potential drugs for the treatment of schizophrenia, as well as other applications.

LY-379,268 is a drug that is used in neuroscience research, which acts as a potent and selective agonist for the group II metabotropic glutamate receptors (mGluR2/3).

LY-487,379 is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It is used to study the structure and function of this receptor subtype, and LY-487,379 along with various other mGluR2/3 agonists and positive modulators are being investigated as possible antipsychotic and anxiolytic drugs.

MGS-0039 is a drug that is used in neuroscientific research, which acts as a potent and selective antagonist for group II of the metabotropic glutamate receptors (mGluR2/3). It produces antidepressant and anxiolytic effects in animal studies, and has been shown to boost release of dopamine and serotonin in specific brain areas. Research has suggested this may occur through a similar mechanism as that suggested for the similarly glutamatergic drug ketamine.

GRN-529 is a drug that was developed by Wyeth as a negative allosteric modulator of the metabotropic glutamate receptor 5 (mGluR5).




















