
CNQX or cyanquixaline (6-cyano-7-nitroquinoxaline-2,3-dione) is a competitive AMPA/kainate receptor antagonist. Its chemical formula is C9H4N4O4. CNQX is often used in the retina to block the responses of OFF-bipolar cells for electrophysiology recordings.

Kainate receptors, or kainic acid receptors (KARs), are ionotropic receptors that respond to the neurotransmitter glutamate. They were first identified as a distinct receptor type through their selective activation by the agonist kainate, a drug first isolated from the algae Digenea simplex. They have been traditionally classified as a non-NMDA-type receptor, along with the AMPA receptor. KARs are less understood than AMPA and NMDA receptors, the other ionotropic glutamate receptors. Postsynaptic kainate receptors are involved in excitatory neurotransmission. Presynaptic kainate receptors have been implicated in inhibitory neurotransmission by modulating release of the inhibitory neurotransmitter GABA through a presynaptic mechanism.

Levocabastine (trade name Livostin or Livocab, depending on the region) is a selective second-generation H1 receptor antagonist which was discovered at Janssen Pharmaceutica in 1979. It is used for allergic conjunctivitis.
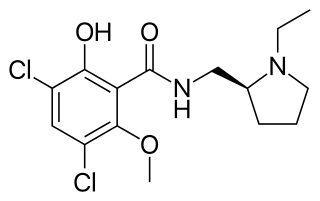
Raclopride is a typical antipsychotic. It acts as a selective antagonist on D2 dopamine receptors. It has been used in trials studying Parkinson Disease.
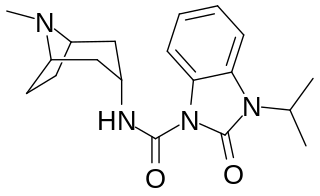
BIMU-8 is a drug which acts as a 5-HT4 receptor selective agonist. BIMU-8 was one of the first compounds of this class. The main action of BIMU-8 is to increase the rate of respiration by activating an area of the brain stem known as the pre-Botzinger complex.

Ifenprodil, sold under the brand names Cerocral, Dilvax, and Vadilex, is a cerebral vasodilator that has been marketed in some countries, including in Japan, Hong Kong, and France. It is currently under development for treatment of a variety of additional indications.

The glutamate receptor, metabotropic 1, also known as GRM1, is a human gene which encodes the metabotropic glutamate receptor 1 (mGluR1) protein.

WAY-100635 is a piperazine drug and research chemical widely used in scientific studies. It was originally believed to act as a selective 5-HT1A receptor antagonist, but subsequent research showed that it also acts as potent full agonist at the D4 receptor. It is sometimes referred to as a silent antagonist at the former receptor. It is closely related to WAY-100135.

Tezampanel is a drug originally developed by Eli Lilly which acts as a competitive antagonist of the AMPA and kainate subtypes of the ionotropic glutamate receptor family, with selectivity for the GluR5 subtype of the kainate receptor. It has neuroprotective and anticonvulsant properties, the former of which may, at least in part, occur via blockade of calcium uptake into neurons.
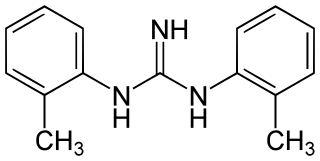
Ditolylguanidine is a sigma receptor agonist. It is somewhat selective for sigma receptors, but non-selective between the two sigma receptor subtypes, binding to both σ1 and σ2 with equal affinity. It has neuroprotective and antidepressant effects, and potentiates the effects of NMDA antagonists.
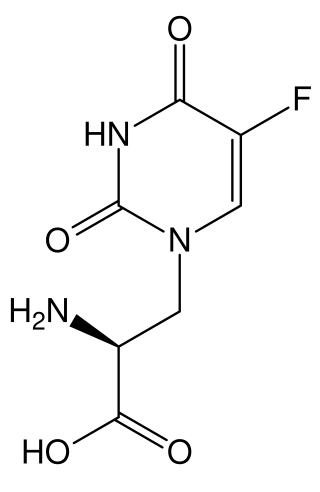
5-Fluorowillardiine is a selective agonist for the AMPA receptor, with only limited effects at the kainate receptor. It is an excitotoxic neurotoxin when used in vivo and so is rarely used in intact animals, but it is widely used to selectively stimulate AMPA receptors in vitro. It is structurally similar to the compound willardiine, which is also an agonist for the AMPA and kainate receptors. Willardiine occurs naturally in Mariosousa willardiana and Acacia sensu lato.

5-Iodowillardiine is a selective agonist for the kainate receptor, with only limited effects at the AMPA receptor. It is selective for kainate receptors composed of GluR5 subunits. It is an excitotoxic neurotoxin in vivo, but has proved highly useful for characterising the subtypes and function of the various kainate receptors in the brain and spinal cord.

Alazocine, also known more commonly as N-allylnormetazocine (NANM), is a synthetic opioid analgesic of the benzomorphan family related to metazocine, which was never marketed. In addition to its opioid activity, the drug is a sigma receptor agonist, and has been used widely in scientific research in studies of this receptor. Alazocine is described as a potent analgesic, psychotomimetic or hallucinogen, and opioid antagonist. Moreover, one of its enantiomers was the first compound that was found to selectively label the σ1 receptor, and led to the discovery and characterization of the receptor.

Dextrallorphan (DXA) is a chemical of the morphinan class that is used in scientific research. It acts as a σ1 receptor agonist and NMDA receptor antagonist. It has no significant affinity for the σ2, μ-opioid, or δ-opioid receptor, or for the serotonin or norepinephrine transporter. As an NMDA receptor antagonist, in vivo, it is approximately twice as potent as dextromethorphan, and five-fold less potent than dextrorphan.
BD1008 or N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-1-pyrrolidineethanamine is a selective sigma receptor antagonist, with a reported binding affinity of Ki = 2 ± 1 nM for the sigma-1 receptor and 4 times selectivity over the sigma-2 receptor.

Hydroxyflutamide (HF, OHF) (developmental code name SCH-16423), or 2-hydroxyflutamide, is a nonsteroidal antiandrogen (NSAA) and the major active metabolite of flutamide, which is considered to be a prodrug of hydroxyflutamide as the active form. It has been reported to possess an IC50 of 700 nM for the androgen receptor (AR), which is about 4-fold less than that of bicalutamide.

Lomerizine (INN) is a diphenylpiperazine class L-type and T-type calcium channel blocker. This drug is currently used clinically for the treatment of migraines, while also being used experimentally for the treatment of glaucoma and optic nerve injury.

WB-4101 is a compound which acts as an antagonist at the α1B-adrenergic receptor. It was one of the first selective antagonists developed for this receptor and was invented in 1969, but is still commonly used in research into adrenergic receptors, especially as a lead compound from which to develop more selective drugs.

PD-137889 (N-methylhexahydrofluorenamine) is a chemical compound that is active as an NMDA receptor antagonist in the central nervous system at roughly 30 times the potency of the "flagship" of its class, ketamine, and substitutes for phencyclidine in animal studies. Ki [3H]TCP binding = 27 nM versus ketamine's Ki = 860 nM.

Willardiine (correctly spelled with two successive i's) or (S)-1-(2-amino-2-carboxyethyl)pyrimidine-2,4-dione is a chemical compound that occurs naturally in the seeds of Mariosousa willardiana and Acacia sensu lato. The seedlings of these plants contain enzymes capable of complex chemical substitutions that result in the formation of free amino acids (See:#Synthesis). Willardiine is frequently studied for its function in higher level plants. Additionally, many derivates of willardiine are researched for their potential in pharmaceutical development. Willardiine was first discovered in 1959 by R. Gmelin, when he isolated several free, non-protein amino acids from Acacia willardiana (another name for Mariosousa willardiana) when he was studying how these families of plants synthesize uracilyalanines. A related compound, Isowillardiine, was concurrently isolated by a different group, and it was discovered that the two compounds had different structural and functional properties. Subsequent research on willardiine has focused on the functional significance of different substitutions at the nitrogen group and the development of analogs of willardiine with different pharmacokinetic properties. In general, Willardiine is the one of the first compounds studied in which slight changes to molecular structure result in compounds with significantly different pharmacokinetic properties.




















