
The metabotropic glutamate receptors, or mGluRs, are a type of glutamate receptor that are active through an indirect metabotropic process. They are members of the group C family of G-protein-coupled receptors, or GPCRs. Like all glutamate receptors, mGluRs bind with glutamate, an amino acid that functions as an excitatory neurotransmitter.
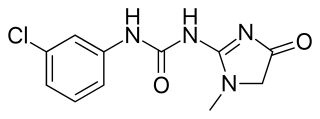
Fenobam is an imidazole derivative developed by McNeil Laboratories in the late 1970s as a novel anxiolytic drug with an at-the-time-unidentified molecular target in the brain. Subsequently, it was determined that fenobam acts as a potent and selective negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5, and it has been used as a lead compound for the development of a range of newer mGluR5 antagonists.

Metabotropic glutamate receptor 2 (mGluR2) is a protein that, in humans, is encoded by the GRM2 gene. mGluR2 is a G protein-coupled receptor (GPCR) that couples with the Gi alpha subunit. The receptor functions as an autoreceptor for glutamate, that upon activation, inhibits the emptying of vesicular contents at the presynaptic terminal of glutamatergic neurons.

Metabotropic glutamate receptor 3 (mGluR3) is an inhibitory Gi/G0-coupled G-protein coupled receptor (GPCR) generally localized to presynaptic sites of neurons in classical circuits. However, in higher cortical circuits in primates, mGluR3 are localized post-synaptically, where they strengthen rather than weaken synaptic connectivity. In humans, mGluR3 is encoded by the GRM3 gene. Deficits in mGluR3 signaling have been linked to impaired cognition in humans, and to increased risk of schizophrenia, consistent with their expanding role in cortical evolution.

Metabotropic glutamate receptor 5 is an excitatory Gq-coupled G protein-coupled receptor predominantly expressed on the postsynaptic sites of neurons. In humans, it is encoded by the GRM5 gene.

Metabotropic glutamate receptor 7 is a protein that in humans is encoded by the GRM7 gene.

Metabotropic glutamate receptor 8 is a protein that in humans is encoded by the GRM8 gene.
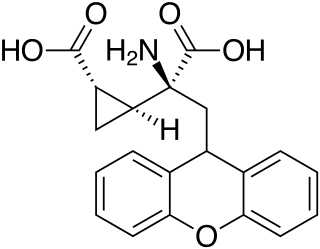
LY-341495 is a research drug developed by the pharmaceutical company Eli Lilly, which acts as a potent and selective orthosteric antagonist for the group II metabotropic glutamate receptors (mGluR2/3).

Biphenylindanone A is a research agent which acts as a potent and selective positive allosteric modulator for the group II metabotropic glutamate receptor subtype mGluR2.

AMN082 is a selective metabotropic glutamate receptor 7 (mGluR7) allosteric agonist. It mimics the effect of glutamate. AMN082 is the first selective mGluR7 agonist and has expanded the potential array of research opportunities on the effects of mGluR7 in the central nervous system.
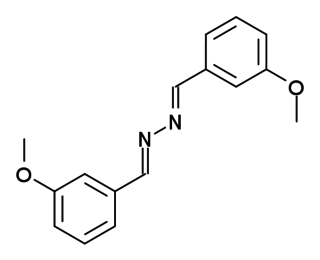
DMeOB is a drug used in scientific research which acts as a negative allosteric modulator of the metabotropic glutamate receptor subtype mGluR5.

PHCCC is a research drug which acts as a glutamate receptor ligand, particularly being a positive allosteric modulator at the mGluR4 subtype, as well as an agonist at mGluR6. It has anxiolytic effects in animal studies. PHCCC and similar drugs have been suggested as novel treatments for Parkinson's disease.

ADX-47273 is a research pharmaceutical developed by Addex Therapeutics which acts as a positive allosteric modulator (PAM) selective for the metabotropic glutamate receptor subtype mGluR5. It has nootropic and antipsychotic effects in animal studies, and has been used as a lead compound to develop improved derivatives.

SIB-1893 is a drug used in scientific research which was one of the first compounds developed that acts as a selective antagonist for the metabotropic glutamate receptor subtype mGluR5. It has anticonvulsant and neuroprotective effects, and reduces glutamate release. It has also been found to act as a positive allosteric modulator of mGluR4.

CDPPB is a drug used in scientific research which acts as a positive allosteric modulator selective for the metabotropic glutamate receptor subtype mGluR5. It has antipsychotic effects in animal models, and mGluR5 modulators are under investigation as potential drugs for the treatment of schizophrenia, as well as other applications.
Ro01-6128 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1. It was derived by modification of a lead compound found via high-throughput screening, and was further developed to give the improved compound Ro67-4853.

Ro67-4853 is a drug used in scientific research, which acts as a selective positive allosteric modulator for the metabotropic glutamate receptor subtype mGluR1. It was derived by modification of the simpler compound Ro01-6128, and has itself subsequently been used as a lead compound to develop a range of potent and selective mGluR1 positive modulators.

LY-487,379 is a drug used in scientific research that acts as a selective positive allosteric modulator for the metabotropic glutamate receptor group II subtype mGluR2. It is used to study the structure and function of this receptor subtype, and LY-487,379 along with various other mGluR2/3 agonists and positive modulators are being investigated as possible antipsychotic and anxiolytic drugs.

VU-0238429 is a drug which acts as a selective positive allosteric modulator for the muscarinic acetylcholine receptor M5. It was the first selective ligand developed for the M5 subtype, and is structurally derived from older M1-selective positive allosteric modulators such as VU-0119498. Replacing the O-methyl- by a phenyl group further improves the receptor subtype selectivity.

RO-4491533 is a drug developed by Hoffmann-La Roche which acts as a potent and selective negative allosteric modulator for group II of the metabotropic glutamate receptors (mGluR2/3), being equipotent at mGluR2 and mGluR3 but without activity at other mGluR subtypes. In animal studies, RO-4491533 produced antidepressant effects and reversed the effects of the mGluR2/3 agonist LY-379,268 with similar efficacy but slightly lower potency than the mGluR2/3 antagonist LY-341,495. A number of related compounds are known, with similar effects in vitro and a fairly well characterized structure-activity relationship.
























