Pro-opiomelanocortin (POMC) is a precursor polypeptide with 241 amino acid residues. POMC is synthesized in corticotrophs of the anterior pituitary from the 267-amino-acid-long polypeptide precursor pre-pro-opiomelanocortin (pre-POMC), by the removal of a 26-amino-acid-long signal peptide sequence during translation. POMC is part of the central melanocortin system.

Secretin is a hormone that regulates water homeostasis throughout the body and influences the environment of the duodenum by regulating secretions in the stomach, pancreas, and liver. It is a peptide hormone produced in the S cells of the duodenum, which are located in the intestinal glands. In humans, the secretin peptide is encoded by the SCT gene.
Appetite is the desire to eat food items, usually due to hunger. Appealing foods can stimulate appetite even when hunger is absent, although appetite can be greatly reduced by satiety. Appetite exists in all higher life-forms, and serves to regulate adequate energy intake to maintain metabolic needs. It is regulated by a close interplay between the digestive tract, adipose tissue and the brain. Appetite has a relationship with every individual's behavior. Appetitive behaviour also known as approach behaviour, and consummatory behaviour, are the only processes that involve energy intake, whereas all other behaviours affect the release of energy. When stressed, appetite levels may increase and result in an increase of food intake. Decreased desire to eat is termed anorexia, while polyphagia is increased eating. Dysregulation of appetite contributes to anorexia nervosa, bulimia nervosa, cachexia, overeating, and binge eating disorder.

Ghrelin is a hormone primarily produced by enteroendocrine cells of the gastrointestinal tract, especially the stomach, and is often called a "hunger hormone" because it increases the drive to eat. Blood levels of ghrelin are highest before meals when hungry, returning to lower levels after mealtimes. Ghrelin may help prepare for food intake by increasing gastric motility and stimulating the secretion of gastric acid.

Neuropeptide Y (NPY) is a 36 amino-acid neuropeptide that is involved in various physiological and homeostatic processes in both the central and peripheral nervous systems. It is secreted alongside other neurotransmitters such as GABA and glutamate.
Satiety is a state or condition of fullness gratified beyond the point of satisfaction, the opposite of hunger. Following satiation, satiety is a feeling of fullness lasting until the next meal. When food is present in the GI tract after a meal, satiety signals overrule hunger signals, but satiety slowly fades as hunger increases.
Glucose-dependent insulinotropic polypeptide, abbreviated as GIP, is an inhibiting hormone of the secretin family of hormones. While it is a weak inhibitor of gastric acid secretion, its main role, being an incretin, is to stimulate insulin secretion.

Amylin, or islet amyloid polypeptide (IAPP), is a 37-residue peptide hormone. It is co-secreted with insulin from the pancreatic β-cells in the ratio of approximately 100:1 (insulin:amylin). Amylin plays a role in glycemic regulation by slowing gastric emptying and promoting satiety, thereby preventing post-prandial spikes in blood glucose levels.

Motilin is a 22-amino acid polypeptide hormone in the motilin family that, in humans, is encoded by the MLN gene.

Obestatin is a hormone that is produced in specialized epithelial cells of the stomach and small intestine of several animals including humans. Obestatin was originally identified as an anorectic peptide, but its effect on food intake remains controversial.
Xenin is a peptide hormone secreted from the chromogranin A-positive enteroendocrine cells called the K-cells in the mucous membrane of the duodenum and stomach of the upper gut. The peptide has been found in humans, dogs, pigs, rats, and rabbits.

Urocortin is a protein that in humans is encoded by the UCN gene. Urocortin belongs to the corticotropin-releasing factor (CRF) family of proteins which includes CRF, urotensin I, sauvagine, urocortin II and urocortin III. Urocortin is involved in the mammalian stress response, and regulates aspects of appetite and stress response.

Glucagon-like peptide-1 (GLP-1) is a 30- or 31-amino-acid-long peptide hormone deriving from the tissue-specific posttranslational processing of the proglucagon peptide. It is produced and secreted by intestinal enteroendocrine L-cells and certain neurons within the nucleus of the solitary tract in the brainstem upon food consumption. The initial product GLP-1 (1–37) is susceptible to amidation and proteolytic cleavage, which gives rise to the two truncated and equipotent biologically active forms, GLP-1 (7–36) amide and GLP-1 (7–37). Active GLP-1 protein secondary structure includes two α-helices from amino acid position 13–20 and 24–35 separated by a linker region.
Neuropeptide Y receptors are a family of receptors belonging to class A G-protein coupled receptors and they are activated by the closely related peptide hormones neuropeptide Y, peptide YY and pancreatic polypeptide. These receptors are involved in the control of a diverse set of behavioral processes including appetite, circadian rhythm, and anxiety.
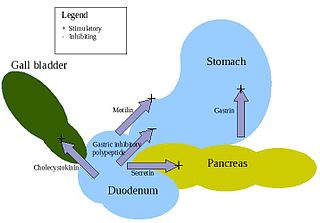
Enteroendocrine cells are specialized cells of the gastrointestinal tract and pancreas with endocrine function. They produce gastrointestinal hormones or peptides in response to various stimuli and release them into the bloodstream for systemic effect, diffuse them as local messengers, or transmit them to the enteric nervous system to activate nervous responses. Enteroendocrine cells of the intestine are the most numerous endocrine cells of the body. They constitute an enteric endocrine system as a subset of the endocrine system just as the enteric nervous system is a subset of the nervous system. In a sense they are known to act as chemoreceptors, initiating digestive actions and detecting harmful substances and initiating protective responses. Enteroendocrine cells are located in the stomach, in the intestine and in the pancreas. Microbiota play key roles in the intestinal immune and metabolic responses in these enteroendocrine cells via their fermentation product, acetate.

The glucagon-like peptide-1 receptor (GLP1R) is a G protein-coupled receptor (GPCR) found on beta cells of the pancreas and on neurons of the brain. It is involved in the control of blood sugar level by enhancing insulin secretion. In humans it is synthesised by the gene GLP1R, which is present on chromosome 6. It is a member of the glucagon receptor family of GPCRs. GLP1R is composed of two domains, one extracellular (ECD) that binds the C-terminal helix of GLP-1, and one transmembrane (TMD) domain that binds the N-terminal region of GLP-1. In the TMD domain there is a fulcrum of polar residues that regulates the biased signaling of the receptor while the transmembrane helical boundaries and extracellular surface are a trigger for biased agonism.

Neuropeptide Y receptor type 1 is a protein that in humans is encoded by the NPY1R gene.

Neuropeptide Y receptor type 2 (Y2R) is a member of the neuropeptide Y receptor family of G-protein coupled receptors, that in humans is encoded by the NPY2R gene.

Pancreatic polypeptide receptor 1, also known as Neuropeptide Y receptor type 4, is a protein that in humans is encoded by the PPYR1 gene.

Teleost leptins are a family of peptide hormones found in fish (teleostei) that are orthologs of the mammalian hormone leptin. The teleost and mammalian leptins appear to have similar functions, namely, regulation of energy intake and expenditure.