
Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.
In viscous fluid dynamics, the Archimedes number (Ar), is a dimensionless number used to determine the motion of fluids due to density differences, named after the ancient Greek scientist and mathematician Archimedes.
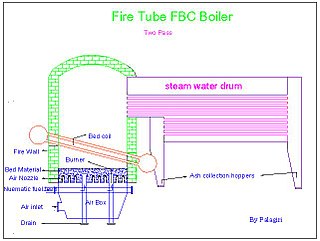
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

Fluidization is a process similar to liquefaction whereby a granular material is converted from a static solid-like state to a dynamic fluid-like state. This process occurs when a fluid is passed up through the granular material.

A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.

A fluidized bed is a physical phenomenon that occurs when a solid particulate substance is under the right conditions so that it behaves like a fluid. The usual way to achieve a fluidized bed is to pump pressurized fluid into the particles. The resulting medium then has many properties and characteristics of normal fluids, such as the ability to free-flow under gravity, or to be pumped using fluid technologies.

In chemical processing, a packed bed is a hollow tube, pipe, or other vessel that is filled with a packing material. The packing can be randomly filled with small objects like Raschig rings or else it can be a specifically designed structured packing. Packed beds may also contain catalyst particles or adsorbents such as zeolite pellets, granular activated carbon, etc.

Fluid Catalytic Cracking (FCC) is the conversion process used in petroleum refineries to convert the high-boiling point, high-molecular weight hydrocarbon fractions of petroleum into gasoline, alkene gases, and other petroleum products. The cracking of petroleum hydrocarbons was originally done by thermal cracking, now virtually replaced by catalytic cracking, which yields greater volumes of high octane rating gasoline; and produces by-product gases, with more carbon-carbon double bonds, that are of greater economic value than the gases produced by thermal cracking.

Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
Self-propagating high-temperature synthesis (SHS) is a method for producing both inorganic and organic compounds by exothermic combustion reactions in solids of different nature. Reactions can occur between a solid reactant coupled with either a gas, liquid, or other solid. If the reactants, intermediates, and products are all solids, it is known as a solid flame. If the reaction occurs between a solid reactant and a gas phase reactant, it is called infiltration combustion. Since the process occurs at high temperatures, the method is ideally suited for the production of refractory materials including powders, metallic alloys, or ceramics.

A bubble column reactor is a chemical reactor that belongs to the general class of multiphase reactors, which consists of three main categories: trickle bed reactor, fluidized bed reactor, and bubble column reactor. A bubble column reactor is a very simple device consisting of a vertical vessel filled with water with a gas distributor at the inlet. Due to the ease of design and operation, which does not involve moving parts, they are widely used in the chemical, biochemical, petrochemical, and pharmaceutical industries to generate and control gas-liquid chemical reactions.
The multiphase particle-in-cell method (MP-PIC) is a numerical method for modeling particle-fluid and particle-particle interactions in a computational fluid dynamics (CFD) calculation. The MP-PIC method achieves greater stability than its particle-in-cell predecessor by simultaneously treating the solid particles as computational particles and as a continuum. In the MP-PIC approach, the particle properties are mapped from the Lagrangian coordinates to an Eulerian grid through the use of interpolation functions. After evaluation of the continuum derivative terms, the particle properties are mapped back to the individual particles. This method has proven to be stable in dense particle flows, computationally efficient, and physically accurate. This has allowed the MP-PIC method to be used as particle-flow solver for the simulation of industrial-scale chemical processes involving particle-fluid flows.

Fluidisation is a phenomenon whereby solid particulate is placed under certain conditions to cause it to behave like a fluid. A fluidized bed is a system conceived to facilitate the fluidisation. Fluidized beds have a wide range of applications including but not limited to: assisting with chemical reactions, heat transfer, mixing and drying. A recent concept devised and patented by Outotec, "An annular fluidized bed consists of a large central nozzle surrounded be a stationary fluidized".
The circulating fluidized bed (CFB) is a type of Fluidized bed combustion that utilizes a recirculating loop for even greater efficiency of combustion. while achieving lower emission of pollutants. Reports suggest that up to 95% of pollutants can be absorbed before being emitted into the atmosphere. The technology is limited in scale however, due to its extensive use of limestone, and the fact that it produces waste byproducts.
As an extension of the fluidized bed family of separation processes, the flash reactor (FR) employs turbulent fluid introduced at high velocities to encourage chemical reactions with feeds and subsequently achieve separation through the chemical conversion of desired substances to different phases and streams. A flash reactor consists of a main reaction chamber and an outlet for separated products to enter downstream processes.
Vibratory Fluidized Bed (VFB) is a type of fluidized bed where the mechanical vibration enhances the performance of fluidization process. Since the first discovery of vibratory fluidized bed, its vibration properties proves to be more efficient in dealing with fine particles which appears to be very difficult to achieve with normal fluidized bed. Even though numerous publications and its popularity in industrial applications, the knowledge about vibratory dynamics and properties are very limited. Future research and development are needed to further improve this technology to bring it to another level.
Calcium looping (CaL), or the regenerative calcium cycle (RCC), is a second-generation carbon capture technology. It is the most developed form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). The captured carbon dioxide can then be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock. Calcium oxide is often referred to as the sorbent.
Heterogenous catalytic reactors put emphasis on catalyst effectiveness factors and the heat and mass transfer implications. Heterogenous catalytic reactors are among the most commonly utilized chemical reactors in the chemical engineering industry.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.










