Syngas, or synthesis gas, is a mixture of hydrogen and carbon monoxide, in various ratios. The gas often contains some carbon dioxide and methane. It is principally used for producing ammonia or methanol. Syngas is combustible and can be used as a fuel. Historically, it has been used as a replacement for gasoline, when gasoline supply has been limited; for example, wood gas was used to power cars in Europe during WWII.

The pyrolysis process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. The word is coined from the Greek-derived elements pyro "fire", "heat", "fever" and lysis "separating".

Gasification is a process that converts biomass- or fossil fuel-based carbonaceous materials into gases, including as the largest fractions: nitrogen (N2), carbon monoxide (CO), hydrogen (H2), and carbon dioxide (CO2). This is achieved by reacting the feedstock material at high temperatures (typically >700 °C), without combustion, via controlling the amount of oxygen and/or steam present in the reaction. The resulting gas mixture is called syngas (from synthesis gas) or producer gas and is itself a fuel due to the flammability of the H2 and CO of which the gas is largely composed. Power can be derived from the subsequent combustion of the resultant gas, and is considered to be a source of renewable energy if the gasified compounds were obtained from biomass feedstock.
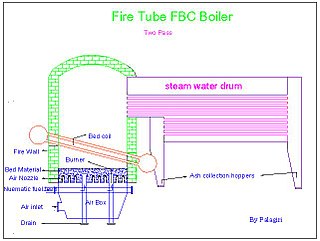
Fluidized bed combustion (FBC) is a combustion technology used to burn solid fuels.

Fluidization is a process similar to liquefaction whereby a granular material is converted from a static solid-like state to a dynamic fluid-like state. This process occurs when a fluid is passed up through the granular material.

The Sabatier reaction or Sabatier process produces methane and water from a reaction of hydrogen with carbon dioxide at elevated temperatures and pressures in the presence of a nickel catalyst. It was discovered by the French chemists Paul Sabatier and Jean-Baptiste Senderens in 1897. Optionally, ruthenium on alumina makes a more efficient catalyst. It is described by the following exothermic reaction:
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable metals. Pyrometallurgical treatment may produce products able to be sold such as pure metals, or intermediate compounds or alloys, suitable as feed for further processing. Examples of elements extracted by pyrometallurgical processes include the oxides of less reactive elements like iron, copper, zinc, chromium, tin, and manganese.
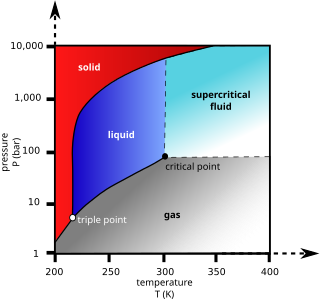
Supercritical carbon dioxide is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.

Carbon capture and storage (CCS) is a process in which a relatively pure stream of carbon dioxide (CO2) from industrial sources is separated, treated and transported to a long-term storage location. For example, the carbon dioxide stream that is to be captured can result from burning fossil fuels or biomass. Usually the CO2 is captured from large point sources, such as a chemical plant or biomass plant, and then stored in an underground geological formation. The aim is to reduce greenhouse gas emissions and thus mitigate climate change. The IPCC's most recent report on mitigating climate change describes CCS retrofits for existing power plants as one of the ways to limit emissions from the electricity sector and meet Paris Agreement goals.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency, compared to conventional pulverized coal combustion.
Hydrogen production is the family of industrial methods for generating hydrogen gas. There are four main sources for the commercial production of hydrogen: natural gas, oil, coal, and electrolysis of water; which account for 48%, 30%, 18% and 4% of the world's hydrogen production respectively. Fossil fuels are the dominant source of industrial hydrogen. As of 2020, the majority of hydrogen (~95%) is produced by steam reforming of natural gas and other light hydrocarbons, partial oxidation of heavier hydrocarbons, and coal gasification. Other methods of hydrogen production include biomass gasification and methane pyrolysis. Methane pyrolysis and water electrolysis can use any source of electricity including renewable energy.
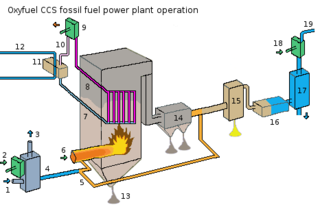
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.
A Direct Carbon Fuel Cell (DCFC) is a fuel cell that uses a carbon rich material as a fuel such as bio-mass or coal. The cell produces energy by combining carbon and oxygen, which releases carbon dioxide as a by-product. It is also called coal fuel cells (CFCs), carbon-air fuel cells (CAFCs), direct carbon/coal fuel cells (DCFCs), and DC-SOFC.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
Hydromethanation, [hahy-droh- meth-uh-ney-shuhn] is the process by which methane is produced through the combination of steam, carbonaceous solids and a catalyst in a fluidized bed reactor. The process, developed over the past 60 years by multiple research groups, enables the highly efficient conversion of coal, petroleum coke and biomass into clean, pipeline quality methane.
The circulating fluidized bed (CFB) is a type of Fluidized bed combustion that utilizes a recirculating loop for even greater efficiency of combustion. while achieving lower emission of pollutants. Reports suggest that up to 95% of pollutants can be absorbed before being emitted into the atmosphere. The technology is limited in scale however, due to its extensive use of limestone, and the fact that it produces waste byproducts.
Calcium looping (CaL), or the regenerative calcium cycle (RCC), is a second-generation carbon capture technology. It is the most developed form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). The captured carbon dioxide can then be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock. Calcium oxide is often referred to as the sorbent.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.
Sorption enhanced water gas shift (SEWGS) is a technology that combines a pre-combustion carbon capture process with the water gas shift reaction (WGS) in order to produce a hydrogen rich stream from the syngas fed to the SEWGS reactor.











