Semiconservative replication describe the mechanism of DNA replication in all known cells. DNA replication occurs on multiple origins of replication along the DNA template strands. As the DNA double helix is unwound by helicase, replication occurs separately on each template strand in antiparallel directions. This process is known as semi-conservative replication because two copies of the original DNA molecule are produced, each copy conserving (replicating) the information from one half of the original DNA molecule. Each copy contains one original strand and one newly synthesized strand. The structure of DNA suggested that each strand of the double helix would serve as a template for synthesis of a new strand. It was not known how newly synthesized strands combined with template strands to form two double helical DNA molecules.

A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create two identical DNA duplexes from a single original DNA duplex. During this process, DNA polymerase "reads" the existing DNA strands to create two new strands that match the existing ones. These enzymes catalyze the chemical reaction
The Meselson–Stahl experiment is an experiment by Matthew Meselson and Franklin Stahl in 1958 which supported Watson and Crick's hypothesis that DNA replication was semiconservative. In semiconservative replication, when the double-stranded DNA helix is replicated, each of the two new double-stranded DNA helices consisted of one strand from the original helix and one newly synthesized. It has been called "the most beautiful experiment in biology." Meselson and Stahl decided the best way to trace the parent DNA would be to tag them by changing one of its atoms. Since nitrogen is present in all of the DNA bases, they generated parent DNA containing a heavier isotope of nitrogen than would be present naturally. This altered mass allowed them to determine how much of the parent DNA was present in the DNA after successive cycles of replication.

Escherichia virus T4 is a species of bacteriophages that infect Escherichia coli bacteria. It is a double-stranded DNA virus in the subfamily Tevenvirinae from the family Myoviridae. T4 is capable of undergoing only a lytic life cycle and not the lysogenic life cycle. The species was formerly named T-even bacteriophage, a name which also encompasses, among other strains, Enterobacteria phage T2, Enterobacteria phage T4 and Enterobacteria phage T6.

Alfred Day Hershey was an American Nobel Prize–winning bacteriologist and geneticist.

Matthew Stanley Meselson is a geneticist and molecular biologist currently at Harvard University, known for his demonstration, with Franklin Stahl, of semi-conservative DNA replication. After completing his Ph.D. under Linus Pauling at the California Institute of Technology, Meselson became a Professor at Harvard University in 1960, where he has remained, today, as Thomas Dudley Cabot Professor of the Natural Sciences.
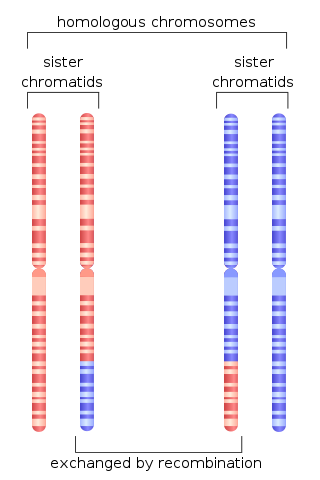
Homologous recombination is a type of genetic recombination in which genetic information is exchanged between two similar or identical molecules of double-stranded or single-stranded nucleic acids.
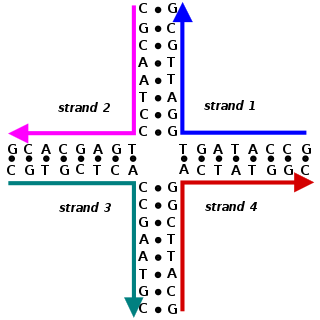
A Holliday junction is a branched nucleic acid structure that contains four double-stranded arms joined. These arms may adopt one of several conformations depending on buffer salt concentrations and the sequence of nucleobases closest to the junction. The structure is named after Robin Holliday, the molecular biologist who proposed its existence in 1964.
Mitotic recombination is a type of genetic recombination that may occur in somatic cells during their preparation for mitosis in both sexual and asexual organisms. In asexual organisms, the study of mitotic recombination is one way to understand genetic linkage because it is the only source of recombination within an individual. Additionally, mitotic recombination can result in the expression of recessive alleles in an otherwise heterozygous individual. This expression has important implications for the study of tumorigenesis and lethal recessive alleles. Mitotic homologous recombination occurs mainly between sister chromatids subsequent to replication. Inter-sister homologous recombination is ordinarily genetically silent. During mitosis the incidence of recombination between non-sister homologous chromatids is only about 1% of that between sister chromatids.
Lethal alleles are alleles that cause the death of the organism that carries them. They are usually a result of mutations in genes that are essential for growth or development. Lethal alleles may be recessive, dominant, or conditional depending on the gene or genes involved.
The phage group was an informal network of biologists centered on Max Delbrück that contributed heavily to bacterial genetics and the origins of molecular biology in the mid-20th century. The phage group takes its name from bacteriophages, the bacteria-infecting viruses that the group used as experimental model organisms. In addition to Delbrück, important scientists associated with the phage group include: Salvador Luria, Alfred Hershey, Seymour Benzer, Charles Steinberg, Gunther Stent, James D. Watson, Frank Stahl, and Renato Dulbecco.
The T4 rII system is an experimental system developed in the 1950s by Seymour Benzer for studying the substructure of the gene. The experimental system is based on genetic crosses of different mutant strains of bacteriophage T4, a virus that infects the bacteria Escherichia coli.
A Chi site or Chi sequence is a short stretch of DNA in the genome of a bacterium near which homologous recombination is more likely to occur than on average across the genome. Chi sites serve as stimulators of DNA double-strand break repair in bacteria, which can arise from radiation or chemical treatments, or result from replication fork breakage during DNA replication. The sequence of the Chi site is unique to each group of closely related organisms; in E. coli and other enteric bacteria, such as Salmonella, the core sequence is 5'-GCTGGTGG-3' plus important nucleotides about 4 to 7 nucleotides to the 3' side of the core sequence. The existence of Chi sites was originally discovered in the genome of bacteriophage lambda, a virus that infects E. coli, but is now known to occur about 1000 times in the E. coli genome.
The NAS Award in Molecular Biology is awarded by the U.S. National Academy of Sciences "for recent notable discovery in molecular biology by a young scientist who is a citizen of the United States." It has been awarded annually since its inception in 1962.
The history of genetics can be represented on a timeline of events from the earliest work in the 1850s, to the DNA era starting in the 1940s, and the genomics era beginning in the 1970s.

Synthesis-dependent strand annealing (SDSA) is a major mechanism of homology-directed repair of DNA double-strand breaks (DSBs). Although many of the features of SDSA were first suggested in 1976, the double-Holliday junction model proposed in 1983 was favored by many researchers. In 1994, studies of double-strand gap repair in Drosophila were found to be incompatible with the double-Holliday junction model, leading researchers to propose a model they called synthesis-dependent strand annealing. Subsequent studies of meiotic recombination in S. cerevisiae found that non-crossover products appear earlier than double-Holliday junctions or crossover products, challenging the previous notion that both crossover and non-crossover products are produced by double-Holliday junctions and leading the authors to propose that non-crossover products are generated through SDSA.
Gisela Mosig was a German-American molecular biologist best known for her work with enterobacteria phage T4. She was among the first investigators to recognize the importance of recombination intermediates in establishing new DNA replication forks, a fundamental process in DNA replication.

Crossover interference is the term used to refer to the non-random placement of crossovers with respect to each other during meiosis. The term is attributed to Hermann Joseph Muller, who observed that one crossover "interferes with the coincident occurrence of another crossing over in the same pair of chromosomes, and I have accordingly termed this phenomenon ‘interference’."

Grete Kellenberger-Gujer (1919–2011) was a Swiss molecular biologist known for her discoveries on genetic recombination and restriction modification system of DNA. She was a pioneer in the genetic analysis of bacteriophages and contributed to the early development of molecular biology.
Charles 'Charley' M. Steinberg was an immunobiologist and permanent member of the Basel Institute for Immunology. He was a former student of Max Delbrück. Notably he hosted Richard Feynman at Caltech when Feynman studied molecular biology, leading Feynman to remark that Charlie was “...the smartest guy I know”. He was instrumental in the discovery of V(D)J recombination, bacteriophage genetics as part of the phage group and co-discoverer of the amber-mutant of the T4 bacteriophage that led to the recognition of stop codons.








