
A chromosome is a long DNA molecule with part or all of the genetic material of an organism. Most eukaryotic chromosomes include packaging proteins called histones which, aided by chaperone proteins, bind to and condense the DNA molecule to maintain its integrity. These chromosomes display a complex three-dimensional structure, which plays a significant role in transcriptional regulation.

In the fields of molecular biology and genetics, a genome is all genetic information of an organism. It consists of nucleotide sequences of DNA. The nuclear genome includes protein-coding genes and non-coding genes, the other functional regions of the genome, and any junk DNA if it is present. Algae and plants contain chloroplasts with a chloroplast genome and almost all eukaryotes have mitochondria and a mitochondrial genome.
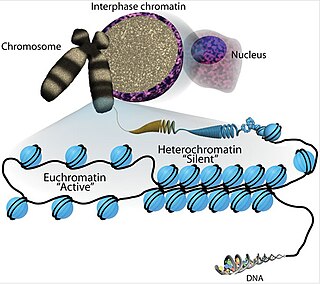
Euchromatin is a lightly packed form of chromatin that is enriched in genes, and is often under active transcription. Euchromatin stands in contrast to heterochromatin, which is tightly packed and less accessible for transcription. 92% of the human genome is euchromatic.
Heterochromatin is a tightly packed form of DNA or condensed DNA, which comes in multiple varieties. These varieties lie on a continuum between the two extremes of constitutive heterochromatin and facultative heterochromatin. Both play a role in the expression of genes. Because it is tightly packed, it was thought to be inaccessible to polymerases and therefore not transcribed; however, according to Volpe et al. (2002), and many other papers since, much of this DNA is in fact transcribed, but it is continuously turned over via RNA-induced transcriptional silencing (RITS). Recent studies with electron microscopy and OsO4 staining reveal that the dense packing is not due to the chromatin.

Gibbons are apes in the family Hylobatidae. The family historically contained one genus, but now is split into four extant genera and 20 species. Gibbons live in subtropical and tropical rainforest from eastern Bangladesh to Northeast India to southern China and Indonesia.
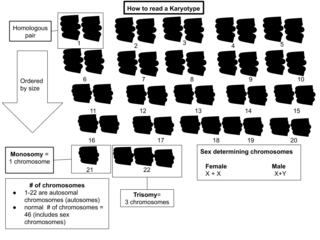
A karyotype is a preparation of the complete set of metaphase chromosomes in the cells of a species or in an individual organism, sorted by length, centromere location and other features and for a test that detects this complement or counts the number of chromosomes. Karyotyping is the process by which a karyotype is prepared from photographs of chromosomes, in order to determine the chromosome complement of an individual, including the number of chromosomes and any abnormalities.

The Y chromosome is one of two sex chromosomes (allosomes) in therian mammals, including humans, and many other animals. The other is the X chromosome. Y is normally the sex-determining chromosome in many species, since it is the presence or absence of Y that determines the male or female sex of offspring produced in sexual reproduction. In mammals, the Y chromosome contains the gene SRY, which triggers male development. The DNA in the human Y chromosome is composed of about 59 million base pairs. The Y chromosome is passed only from father to son. With a 30% difference between humans and chimpanzees, the Y chromosome is one of the fastest-evolving parts of the human genome. The human Y chromosome carries an estimated 100-200 genes, with between 45 and 73 of these being protein-coding. All single-copy Y-linked genes are hemizygous except in cases of aneuploidy such as XYY syndrome or XXYY syndrome.

Afrotheria is a clade of mammals, the living members of which belong to groups that are either currently living in Africa or of African origin: golden moles, elephant shrews, tenrecs, aardvarks, hyraxes, elephants, sea cows, and several extinct clades. Most groups of afrotheres share little or no superficial resemblance, and their similarities have only become known in recent times because of genetics and molecular studies. Many afrothere groups are found mostly or exclusively in Africa, reflecting the fact that Africa was an island continent from the Cretaceous until the early Miocene around 20 million years ago, when Afro-Arabia collided with Eurasia.

Cytogenetics is essentially a branch of genetics, but is also a part of cell biology/cytology, that is concerned with how the chromosomes relate to cell behaviour, particularly to their behaviour during mitosis and meiosis. Techniques used include karyotyping, analysis of G-banded chromosomes, other cytogenetic banding techniques, as well as molecular cytogenetics such as fluorescent in situ hybridization (FISH) and comparative genomic hybridization (CGH).

Euarchontoglires is a clade and a superorder of mammals, the living members of which belong to one of the five following groups: rodents, lagomorphs, treeshrews, colugos and primates.
The Euarchonta are a proposed grandorder of mammals: the order Scandentia (treeshrews), and its sister Primatomorpha mirorder, containing the Dermoptera or colugos and the primates.
Comparative genomic hybridization(CGH) is a molecular cytogenetic method for analysing copy number variations (CNVs) relative to ploidy level in the DNA of a test sample compared to a reference sample, without the need for culturing cells. The aim of this technique is to quickly and efficiently compare two genomic DNA samples arising from two sources, which are most often closely related, because it is suspected that they contain differences in terms of either gains or losses of either whole chromosomes or subchromosomal regions. This technique was originally developed for the evaluation of the differences between the chromosomal complements of solid tumor and normal tissue, and has an improved resolution of 5–10 megabases compared to the more traditional cytogenetic analysis techniques of giemsa banding and fluorescence in situ hybridization (FISH) which are limited by the resolution of the microscope utilized.
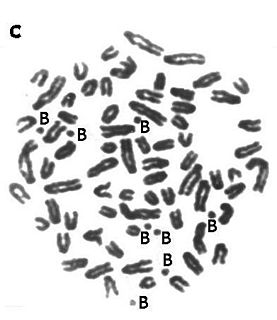
In addition to the normal karyotype, wild populations of many animal, plant, and fungi species contain B chromosomes. By definition, these chromosomes are not essential for the life of a species, and are lacking in some of the individuals. Thus a population would consist of individuals with 0, 1, 2, 3 (etc.) B chromosomes. B chromosomes are distinct from marker chromosomes or additional copies of normal chromosomes as they occur in trisomies.

Polysomy is a condition found in many species, including fungi, plants, insects, and mammals, in which an organism has at least one more chromosome than normal, i.e., there may be three or more copies of the chromosome rather than the expected two copies. Most eukaryotic species are diploid, meaning they have two sets of chromosomes, whereas prokaryotes are haploid, containing a single chromosome in each cell. Aneuploids possess chromosome numbers that are not exact multiples of the haploid number and polysomy is a type of aneuploidy. A karyotype is the set of chromosomes in an organism and the suffix -somy is used to name aneuploid karyotypes. This is not to be confused with the suffix -ploidy, referring to the number of complete sets of chromosomes.

Boreoeutheria is a magnorder of placental mammals that groups together superorders Euarchontoglires and Laurasiatheria. With a few exceptions male animals in the clade have a scrotum, an ancestral feature of the clade. The sub-clade Scrotifera was named after this feature.

Molecular cytogenetics combines two disciplines, molecular biology and cytogenetics, and involves the analysis of chromosome structure to help distinguish normal and cancer-causing cells. Human cytogenetics began in 1956 when it was discovered that normal human cells contain 46 chromosomes. However, the first microscopic observations of chromosomes were reported by Arnold, Flemming, and Hansemann in the late 1800s. Their work was ignored for decades until the actual chromosome number in humans was discovered as 46. In 1879, Arnold examined sarcoma and carcinoma cells having very large nuclei. Today, the study of molecular cytogenetics can be useful in diagnosing and treating various malignancies such as hematological malignancies, brain tumors, and other precursors of cancer. The field is overall focused on studying the evolution of chromosomes, more specifically the number, structure, function, and origin of chromosome abnormalities. It includes a series of techniques referred to as fluorescence in situ hybridization, or FISH, in which DNA probes are labeled with different colored fluorescent tags to visualize one or more specific regions of the genome. Introduced in the 1980s, FISH uses probes with complementary base sequences to locate the presence or absence of the specific DNA regions you are looking for. FISH can either be performed as a direct approach to metaphase chromosomes or interphase nuclei. Alternatively, an indirect approach can be taken in which the entire genome can be assessed for copy number changes using virtual karyotyping. Virtual karyotypes are generated from arrays made of thousands to millions of probes, and computational tools are used to recreate the genome in silico.
Virtual karyotype is the digital information reflecting a karyotype, resulting from the analysis of short sequences of DNA from specific loci all over the genome, which are isolated and enumerated. It detects genomic copy number variations at a higher resolution for level than conventional karyotyping or chromosome-based comparative genomic hybridization (CGH). The main methods used for creating virtual karyotypes are array-comparative genomic hybridization and SNP arrays.

A microchromosome (μChr) is a type of very small chromosome which is a typical component of the karyotype of birds, some reptiles, fish, and amphibians; they have yet to found in mammals. They are less than 20 Mb in size; chromosomes which are greater than 40 Mb in size are known as macrochromosomes (MChrs), while those between 20 and 40 Mb are classified as intermediate chromosomes. Microchromosomes are characteristically very small and often cytogenetically indistinguishable in a karyotype.
Holocentric chromosomes are chromosomes that possess multiple kinetochores along their length rather than the single centromere typical of other chromosomes. They were first described in cytogenetic experiments in 1935. Since this first observation, the term holocentric chromosome has referred to chromosomes that: i) lack the primary constriction corresponding to the centromere observed in monocentric chromosomes; and ii) possess multiple kinetochores dispersed along the entire chromosomal axis, such that microtubules bind to the chromosome along its entire length and move broadside to the pole from the metaphase plate. Holocentric chromosomes are also termed holokinetic, because, during cell division, the sister chromatids move apart in parallel and do not form the classical V-shaped figures typical of monocentric chromosomes.


















