
The lymphatic system, or lymphoid system, is an organ system in vertebrates that is part of the immune system, and complementary to the circulatory system. It consists of a large network of lymphatic vessels, lymph nodes, lymphoid organs, lymphoid tissues and lymph. Lymph is a clear fluid carried by the lymphatic vessels back to the heart for re-circulation..
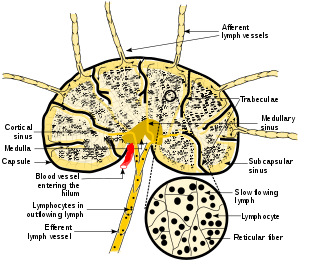
A lymph node, or lymph gland, is a kidney-shaped organ of the lymphatic system and the adaptive immune system. A large number of lymph nodes are linked throughout the body by the lymphatic vessels. They are major sites of lymphocytes that include B and T cells. Lymph nodes are important for the proper functioning of the immune system, acting as filters for foreign particles including cancer cells, but have no detoxification function.

A dendritic cell (DC) is an antigen-presenting cell of the mammalian immune system. A DC's main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and adaptive immune systems.

Macrophages are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris, and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This process is called phagocytosis, which acts to defend the host against infection and injury.

Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae. Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery. Phagocytes occur in many species; some amoebae behave like macrophage phagocytes, which suggests that phagocytes appeared early in the evolution of life.

Monocytes are a type of leukocyte or white blood cell. They are the largest type of leukocyte in blood and can differentiate into macrophages and monocyte-derived dendritic cells. As a part of the vertebrate innate immune system monocytes also influence adaptive immune responses and exert tissue repair functions. There are at least three subclasses of monocytes in human blood based on their phenotypic receptors.
In immunology, the mononuclear phagocyte system or mononuclear phagocytic system (MPS) also known as the reticuloendothelial system or macrophage system is a part of the immune system that consists of the phagocytic cells located in reticular connective tissue. The cells are primarily monocytes and macrophages, and they accumulate in lymph nodes and the spleen. The Kupffer cells of the liver and tissue histiocytes are also part of the MPS. The mononuclear phagocyte system and the monocyte macrophage system refer to two different entities, often mistakenly understood as one.
A histiocyte is a vertebrate cell that is part of the mononuclear phagocyte system. The mononuclear phagocytic system is part of the organism's immune system. The histiocyte is a tissue macrophage or a dendritic cell. Part of their job is to clear out neutrophils once they've reached the end of their lifespan.

An antigen-presenting cell (APC) or accessory cell is a cell that displays antigen bound by major histocompatibility complex (MHC) proteins on its surface; this process is known as antigen presentation. T cells may recognize these complexes using their T cell receptors (TCRs). APCs process antigens and present them to T-cells.
Gut-associated lymphoid tissue (GALT) is a component of the mucosa-associated lymphoid tissue (MALT) which works in the immune system to protect the body from invasion in the gut.
Malignant histiocytosis is a rare hereditary disease found in the Bernese Mountain Dog and humans, characterized by histiocytic infiltration of the lungs and lymph nodes. The liver, spleen, and central nervous system can also be affected. Histiocytes are a component of the immune system that proliferate abnormally in this disease. In addition to its importance in veterinary medicine, the condition is also important in human pathology.
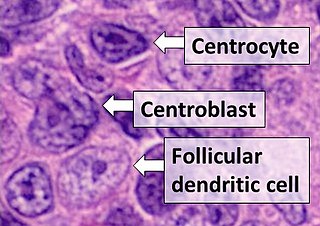
Follicular dendritic cells (FDC) are cells of the immune system found in primary and secondary lymph follicles of the B cell areas of the lymphoid tissue. Unlike dendritic cells (DC), FDCs are not derived from the bone-marrow hematopoietic stem cell, but are of mesenchymal origin. Possible functions of FDC include: organizing lymphoid tissue's cells and microarchitecture, capturing antigen to support B cell, promoting debris removal from germinal centers, and protecting against autoimmunity. Disease processes that FDC may contribute include primary FDC-tumor, chronic inflammatory conditions, HIV-1 infection development, and neuroinvasive scrapie.

Monoblasts are the committed progenitor cells that differentiated from a committed macrophage or dendritic cell precursor (MDP) in the process of hematopoiesis. They are the first developmental stage in the monocyte series leading to a macrophage. Their myeloid cell fate is induced by the concentration of cytokines they are surrounded by during development. These cytokines induce the activation of transcription factors which push completion of the monoblast's myeloid cell fate. Monoblasts are normally found in bone marrow and do not appear in the normal peripheral blood. They mature into monocytes which, in turn, develop into macrophages. They then are seen as macrophages in the normal peripheral blood and many different tissues of the body. Macrophages can produce a variety of effector molecules that initiate local, systemic inflammatory responses. These monoblast differentiated cells are equipped to fight off foreign invaders using pattern recognition receptors to detect antigen as part of the innate immune response.
Certain sites of the mammalian body have immune privilege, meaning they are able to tolerate the introduction of antigens without eliciting an inflammatory immune response. Tissue grafts are normally recognised as foreign antigens by the body and attacked by the immune system. However, in immune privileged sites, tissue grafts can survive for extended periods of time without rejection occurring. Immunologically privileged sites include:
In immunology, peripheral tolerance is the second branch of immunological tolerance, after central tolerance. It takes place in the immune periphery. Its main purpose is to ensure that self-reactive T and B cells which escaped central tolerance do not cause autoimmune disease. Peripheral tolerance prevents immune response to harmless food antigens and allergens, too.

C-C chemokine receptor type 7 is a protein that in humans is encoded by the CCR7 gene. Two ligands have been identified for this receptor: the chemokines ligand 19 (CCL19/ELC) and ligand 21 (CCL21). The ligands have similar affinity for the receptor, though CCL19 has been shown to induce internalisation of CCR7 and desensitisation of the cell to CCL19/CCL21 signals. CCR7 is a transmembrane protein with 7 transmembrane domains, which is coupled with heterotrimeric G proteins, which transduce the signal downstream through various signalling cascades. The main function of the receptor is to guide immune cells to immune organs by detecting specific chemokines, which these tissues secrete.

G-protein coupled receptor 183 also known as Epstein-Barr virus-induced G-protein coupled receptor 2 (EBI2) is a protein (GPCR) expressed on the surface of some immune cells, namely B cells and T cells; in humans it is encoded by the GPR183 gene. Expression of EBI2 is one critical mediator of immune cell localization within lymph nodes, responsible in part for the coordination of B cell, T cell, and dendritic cell movement and interaction following antigen exposure. EBI2 is a receptor for oxysterols. The most potent activator is 7α,25-dihydroxycholesterol (7α,25-OHC), with other oxysterols exhibiting varying affinities for the receptor. Oxysterol gradients drive chemotaxis, attracting the EBI2-expressing cells to locations of high ligand concentration. The GPR183 gene was identified due to its upregulation during Epstein-Barr virus infection of the Burkitt's lymphoma cell line BL41, hence its name: EBI2.
Lymph node stromal cells are essential to the structure and function of the lymph node whose functions include: creating an internal tissue scaffold for the support of hematopoietic cells; the release of small molecule chemical messengers that facilitate interactions between hematopoietic cells; the facilitation of the migration of hematopoietic cells; the presentation of antigens to immune cells at the initiation of the adaptive immune system; and the homeostasis of lymphocyte numbers. Stromal cells originate from multipotent mesenchymal stem cells.
Miram Merad is a French-Algerian professor in Cancer immunology and the Director of the Marc and Jennifer Lipschultz Precision Immunology Institute (PrIISM) at the Icahn School of Medicine at Mount Sinai (ISMMS) in New York, NY. She is the corecipient of the 2018 William B. Coley Award for Distinguished Research in Basic Immunology and a member of the United States National Academy of Sciences and the National Academy of Medicine.
Melanie Greter is a Swiss neuroimmunologist and a Swiss National Science Foundation Professor in the Institute of Experimental Immunology at the University of Zurich. Greter explores the ontogeny and function of microglia and border-associated macrophages of the central nervous system to understand how they maintain homeostasis and contribute to brain-related diseases.











