
Allotropy or allotropism is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: the atoms of the element are bonded together in different manners. For example, the allotropes of carbon include diamond, graphite, graphene, and fullerenes.

Carbon is a chemical element; it has symbol C and atomic number 6. It is nonmetallic and tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 electrons. It belongs to group 14 of the periodic table. Carbon makes up about 0.025 percent of Earth's crust. Three isotopes occur naturally, 12C and 13C being stable, while 14C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the few elements known since antiquity.

A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometer range (nanoscale). They are one of the allotropes of carbon.

A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to seven atoms. The molecules may be hollow spheres, ellipsoids, tubes, or other shapes.
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds—that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as inorganic chemistry.

Carbon is capable of forming many allotropes due to its valency. Well-known forms of carbon include diamond and graphite. In recent decades, many more allotropes have been discovered and researched, including ball shapes such as buckminsterfullerene and sheets such as graphene. Larger-scale structures of carbon include nanotubes, nanobuds and nanoribbons. Other unusual forms of carbon exist at very high temperatures or extreme pressures. Around 500 hypothetical 3‑periodic allotropes of carbon are known at the present time, according to the Samara Carbon Allotrope Database (SACADA).
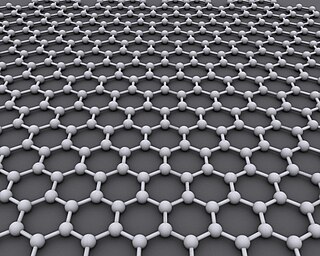
Graphene is an allotrope of carbon consisting of a single layer of atoms arranged in a hexagonal lattice nanostructure. The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds.

Aggregated diamond nanorods, or ADNRs, are a nanocrystalline form of diamond, also known as nanodiamond or hyperdiamond.

A polyyne is any organic compound with alternating single and triple bonds; that is, a series of consecutive alkynes, (−C≡C−)n with n greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds (−CR=CR′−)n with n greater than 1. They are also sometimes referred to as oligoynes, or carbinoids after "carbyne" (−C≡C−)∞, the hypothetical allotrope of carbon that would be the ultimate member of the series. The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today.

The element sulfur exists as many allotropes. In number of allotropes, sulfur is second only to carbon. In addition to the allotropes, each allotrope often exists in polymorphs delineated by Greek prefixes.

Biphenylene is an organic compound with the formula (C6H4)2. It is a pale, yellowish solid with a hay-like odor. Despite its unusual structure, it behaves like a traditional polycyclic aromatic hydrocarbon.

Elemental phosphorus can exist in several allotropes, the most common of which are white and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.

Linear acetylenic carbon (LAC), also known as carbyne or Linear Carbon Chain (LCC), is an allotrope of carbon that has the chemical structure (−C≡C−)n as a repeat unit, with alternating single and triple bonds. It would thus be the ultimate member of the polyyne family.
In organic chemistry, a cyclo[n]carbon is a chemical compound consisting solely of a number n of carbon atoms covalently linked in a ring. Since the compounds are composed only of carbon atoms, they are allotropes of carbon. Possible bonding patterns include all double bonds or alternating single bonds and triple bonds.
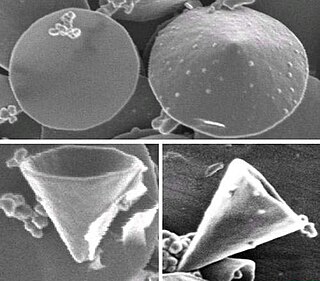
Carbon nanocones are conical structures which are made predominantly from carbon and which have at least one dimension of the order one micrometer or smaller. Nanocones have height and base diameter of the same order of magnitude; this distinguishes them from tipped nanowires which are much longer than their diameter. Nanocones occur on the surface of natural graphite. Hollow carbon nanocones can also be produced by decomposing hydrocarbons with a plasma torch. Electron microscopy reveals that the opening angle (apex) of the cones is not arbitrary, but has preferred values of approximately 20°, 40°, and 60°. This observation was explained by a model of the cone wall composed of wrapped graphene sheets, where the geometrical requirement for seamless connection naturally accounted for the semi-discrete character and the absolute values of the cone angle. A related carbon nanoform is the single-walled carbon nanohorn which typically form aggregates 80–100 nm in size.
Superdense carbon allotropes are proposed configurations of carbon atoms that result in a stable material with a higher density than diamond.
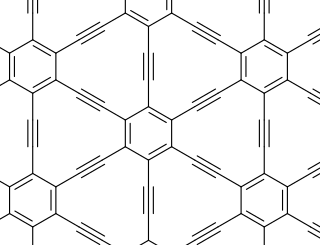
Graphyne is an allotrope of carbon. Its structure is one-atom-thick planar sheets of sp and sp2-bonded carbon atoms arranged in crystal lattice. It can be seen as a lattice of benzene rings connected by acetylene bonds. The material is called graphyne-n when benzene rings are connected by n sequential acetylene molecules, and graphdiyne for a particular case of n = 2.

Penta-graphene is a hypothetical carbon allotrope composed entirely of carbon pentagons and resembling the Cairo pentagonal tiling. Penta-graphene was proposed in 2014 on the basis of analyses and simulations. Further calculations predicted that it is unstable in its pure form, but can be stabilized by hydrogenation. Due to its atomic configuration, penta-graphene has an unusually negative Poisson’s ratio and very high ideal strength believed to exceed that of a similar material, graphene.
Phagraphene [fæ’græfiːn] is a proposed graphene allotrope composed of 5-6-7 carbon rings. Phagraphene was proposed in 2015 based on systematic evolutionary structure searching. Theoretical calculations showed that phagraphene is not only dynamically and thermally stable, but also has distorted Dirac cones. The direction-dependent cones are robust against external strain with tuneable Fermi velocities.

Cyclooctadeca-1,3,5,7,9,11,13,15,17-nonayne or cyclo[18]carbon is an allotrope of carbon with molecular formula C
18. The molecule is a ring of eighteen carbon atoms, connected by alternating triple and single bonds; thus, it is a polyyne and a cyclocarbon.

















