

Various nitrogen compounds were made from hartshorn shavings:
Hartshorn jelly or a decoction of burnt hartshorn in water was used to treat diarrhea. The coal of hartshorn, called calcinated hartshorn, was used as an absorbent, as well as in the treatment of dysentery. Salt of hartshorn (ammonium carbonate) was used as a sudorific for treatment of fevers, and as a smelling salt. [2] Hartshorn was used to treat insect bites, [3] sunstroke, [4] stye, [5] and snakebites. [6]
Hartshorn salt (ammonium carbonate), also known simply as hartshorn, and baker's ammonia, was used as a leavening agent, in the baking of cookies and other edible treats. It was used mainly in the seventeenth and eighteenth centuries as a forerunner of baking powder. [7] A half-teaspoon of hartshorn can substitute for one teaspoon of baking powder. It is called for in old German and Scandinavian recipes and, although rarely used in modern times, may still be purchased as a baking ingredient. Hartshorn helps molded cookies such as Springerle to retain their intricate designs during baking. Cookies made with hartshorn can be kept for a long time without hardening. Use of hartshorn may turn some ingredients, such as sunflower seeds, green.
Ammonium carbonate is especially suited to thin, dry cookies and crackers. When heated, it releases ammonia and carbon dioxide gases, but no water. The absence of water allows cookies to cook and dry out more quickly, and thinner cookies allow the pungent ammonia to escape, rather than to remain trapped, as it would in a deeper mass.
Ammonia released during the baking process reacts with glucose and fructose to form intermediate molecules that in turn, react with asparagine (an amino acid found in nuts, seeds, and whole grains) to form acrylamide, a carcinogen. [8] [9]

Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the formula NH3. A stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to fertilisers. Around 70% of ammonia produced industrially is used to make fertilisers in various forms and composition, such as urea and diammonium phosphate. Ammonia in pure form is also applied directly into the soil.

In chemistry, there are three definitions in common use of the word "base": Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century.

Sodium carbonate is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood, sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.

Baking powder is a dry chemical leavening agent, a mixture of a carbonate or bicarbonate and a weak acid. The base and acid are prevented from reacting prematurely by the inclusion of a buffer such as cornstarch. Baking powder is used to increase the volume and lighten the texture of baked goods. It works by releasing carbon dioxide gas into a batter or dough through an acid–base reaction, causing bubbles in the wet mixture to expand and thus leavening the mixture. The first single-acting baking powder, which releases carbon dioxide at room temperature as soon as it is dampened, was developed by food manufacturer Alfred Bird in England in 1843. The first double-acting baking powder, which releases some carbon dioxide when dampened, and later releases more of the gas when heated by baking, was first developed by Eben Norton Horsford in the U.S. in the 1860s.
Potassium carbonate is the inorganic compound with the formula K2CO3. It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass.

Ammonium chloride is an inorganic compound with the formula NH4Cl and a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. In its naturally occurring mineralogic form, it is known as sal ammoniac. The mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. It is also found around some types of volcanic vents. It is mainly used as fertilizer and a flavouring agent in some types of liquorice. It is the product from the reaction of hydrochloric acid and ammonia.

Salammoniac, also sal ammoniac or salmiac, is a rare naturally occurring mineral composed of ammonium chloride, NH4Cl. It forms colorless, white, or yellow-brown crystals in the isometric-hexoctahedral class. It has very poor cleavage and is brittle to conchoidal fracture. It is quite soft, with a Mohs hardness of 1.5 to 2, and it has a low specific gravity of 1.5. It is water-soluble. Sal ammoniac is also the archaic name for the chemical compound ammonium chloride.

Ammonium bicarbonate is an inorganic compound with formula (NH4)HCO3. The compound has many names, reflecting its long history. Chemically speaking, it is the bicarbonate salt of the ammonium ion. It is a colourless solid that degrades readily to carbon dioxide, water and ammonia.
Ammonia solution, also known as ammonia water, ammonium hydroxide, ammoniacal liquor, ammonia liquor, aqua ammonia, aqueous ammonia, or (inaccurately) ammonia, is a solution of ammonia in water. It can be denoted by the symbols NH3(aq). Although the name ammonium hydroxide suggests an alkali with the composition [NH+
4][OH−
], it is actually impossible to isolate samples of NH4OH. The ions NH+
4 and OH− do not account for a significant fraction of the total amount of ammonia except in extremely dilute solutions.
The Solvay process or ammonia-soda process is the major industrial process for the production of sodium carbonate (soda ash, Na2CO3). The ammonia-soda process was developed into its modern form by the Belgian chemist Ernest Solvay during the 1860s. The ingredients for this are readily available and inexpensive: salt brine (from inland sources or from the sea) and limestone (from quarries). The worldwide production of soda ash in 2005 was estimated at 42 million tonnes, which is more than six kilograms (13 lb) per year for each person on Earth. Solvay-based chemical plants now produce roughly three-quarters of this supply, with the remaining being mined from natural deposits. This method superseded the Leblanc process.

In organic chemistry, a carbamate is a category of organic compounds with the general formula R2NC(O)OR and structure >N−C(=O)−O−, which are formally derived from carbamic acid. The term includes organic compounds, formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H2NCOO−.

Smelling salts, also known as ammonia inhalants, spirit of hartshorne or sal volatile, are chemical compounds used as stimulants to restore consciousness after fainting.

Ammonium carbonate is a salt with the chemical formula (NH4)2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and is a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn, and produces a pungent smell when baked.
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes.
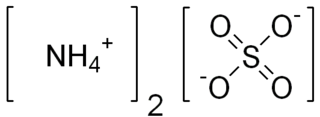
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.
Acid salts are a class of salts that produce an acidic solution after being dissolved in a solvent. Its formation as a substance has a greater electrical conductivity than that of the pure solvent. An acidic solution formed by acid salt is made during partial neutralization of diprotic or polyprotic acids. A half-neutralization occurs due to the remaining of replaceable hydrogen atoms from the partial dissociation of weak acids that have not been reacted with hydroxide ions to create water molecules.
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. It is also abbreviated TEA, yet this abbreviation must be used carefully to avoid confusion with triethanolamine or tetraethylammonium, for which TEA is also a common abbreviation. It is a colourless volatile liquid with a strong fishy odor reminiscent of ammonia. Like diisopropylethylamine (Hünig's base), triethylamine is commonly employed in organic synthesis, usually as a base.

Springerle is a type of South German biscuit or cookie with an embossed design made by pressing a mold onto rolled dough and allowing the impression to dry before baking. This preserves the detail of the surface pattern. While historical molds show that springerle were baked for religious holidays and secular occasions throughout the year, they are now most commonly associated with the Christmas season.
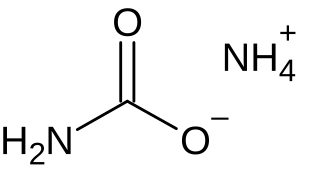
Ammonium carbamate is a chemical compound with the formula [NH4][H2NCO2] consisting of ammonium cation NH+4 and carbamate anion NH2COO−. It is a white solid that is extremely soluble in water, less so in alcohol. Ammonium carbamate can be formed by the reaction of ammonia NH3 with carbon dioxide CO2, and will slowly decompose to those gases at ordinary temperatures and pressures. It is an intermediate in the industrial synthesis of urea (NH2)2CO, an important fertilizer.