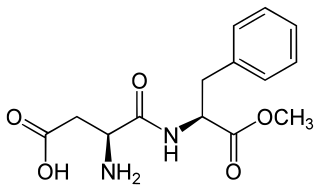
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Genetic engineering, also called genetic modification or genetic manipulation, is the modification and manipulation of an organism's genes using technology. It is a set of technologies used to change the genetic makeup of cells, including the transfer of genes within and across species boundaries to produce improved or novel organisms.
The Monsanto Company was an American agrochemical and agricultural biotechnology corporation founded in 1901 and headquartered in Creve Coeur, Missouri. Monsanto's best-known product is Roundup, a glyphosate-based herbicide, developed in the 1970s. Later, the company became a major producer of genetically engineered crops. In 2018, the company ranked 199th on the Fortune 500 of the largest United States corporations by revenue.

The Hoover Institution is an American public policy think tank which promotes personal and economic liberty, free enterprise, and limited government. While the institution is formally a unit of Stanford University, it maintains an independent board of overseers and relies on its own income and donations. It is widely described as conservative, although its directors have contested the idea that it is partisan.
Genentech, Inc. is an American biotechnology corporation headquartered in South San Francisco, California. It became an independent subsidiary of Roche in 2009. Genentech Research and Early Development operates as an independent center within Roche. Historically, the company is regarded as the world's first biotechnology company.

Bovine somatotropin or bovine somatotrophin, or bovine growth hormone (BGH), is a peptide hormone produced by cows' pituitary glands. Like other hormones, it is produced in small quantities and is used in regulating metabolic processes. Scientists created a bacterium that produces the hormone somatotropin which is produced by the cow's body after giving birth and increases milk production by around 10 percent.
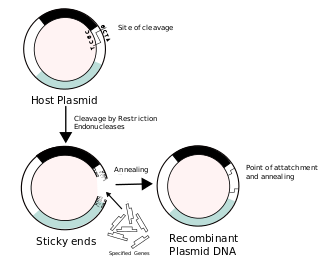
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome.
Pharming, a portmanteau of farming and pharmaceutical, refers to the use of genetic engineering to insert genes that code for useful pharmaceuticals into host animals or plants that would otherwise not express those genes, thus creating a genetically modified organism (GMO). Pharming is also known as molecular farming, molecular pharming, or biopharming.

Robert Samuel Langer Jr. FREng is an American biotechnologist, businessman, chemical engineer, chemist, and inventor. He is one of the nine Institute Professors at the Massachusetts Institute of Technology.

Genetically modified crops are plants used in agriculture, the DNA of which has been modified using genetic engineering methods. Plant genomes can be engineered by physical methods or by use of Agrobacterium for the delivery of sequences hosted in T-DNA binary vectors. In most cases, the aim is to introduce a new trait to the plant which does not occur naturally in the species. Examples in food crops include resistance to certain pests, diseases, environmental conditions, reduction of spoilage, resistance to chemical treatments, or improving the nutrient profile of the crop. Examples in non-food crops include production of pharmaceutical agents, biofuels, and other industrially useful goods, as well as for bioremediation.

Genetically modified food controversies are disputes over the use of foods and other goods derived from genetically modified crops instead of conventional crops, and other uses of genetic engineering in food production. The disputes involve consumers, farmers, biotechnology companies, governmental regulators, non-governmental organizations, and scientists. The key areas of controversy related to genetically modified food are whether such food should be labeled, the role of government regulators, the objectivity of scientific research and publication, the effect of genetically modified crops on health and the environment, the effect on pesticide resistance, the impact of such crops for farmers, and the role of the crops in feeding the world population. In addition, products derived from GMO organisms play a role in the production of ethanol fuels and pharmaceuticals.

APCO Worldwide is an independent global public affairs and strategic communications consultancy. With 680 employees in 35 worldwide locations, it is also the fifth largest independently owned PR firm in the United States. Headquartered in Washington, D.C., APCO was founded in 1984 by Margery Kraus, who is now the firm's Executive Chairman.
The United States is the largest grower of commercial crops that have been genetically engineered in the world, but not without domestic and international opposition.

Bayer AG is a German multinational pharmaceutical and biotechnology company and is one of the largest pharmaceutical companies and biomedical companies in the world. Headquartered in Leverkusen, Bayer's areas of business include: pharmaceuticals, consumer healthcare products, agricultural chemicals, seeds and biotechnology products. The company is a component of the EURO STOXX 50 stock market index.
Ultralente insulin was a long-acting form of insulin. It has an onset of 4 to 6 hours, a peak of 14 to 24 hours, and a duration of 28 to 36 hours. Ultralente insulin, along with lente insulin, were discontinued in the US by manufacturers in the mid-2000s. One of the reasons for discontinuation was declining use in favor of NPH insulin and other newer insulin products. The FDA withdrew approval for ultralente insulin products by 2011.

Genetic engineering is the science of manipulating genetic material of an organism. The first artificial genetic modification accomplished using biotechnology was transgenesis, the process of transferring genes from one organism to another, first accomplished by Herbert Boyer and Stanley Cohen in 1973. It was the result of a series of advancements in techniques that allowed the direct modification of the genome. Important advances included the discovery of restriction enzymes and DNA ligases, the ability to design plasmids and technologies like polymerase chain reaction and sequencing. Transformation of the DNA into a host organism was accomplished with the invention of biolistics, Agrobacterium-mediated recombination and microinjection. The first genetically modified animal was a mouse created in 1974 by Rudolf Jaenisch. In 1976 the technology was commercialised, with the advent of genetically modified bacteria that produced somatostatin, followed by insulin in 1978. In 1983 an antibiotic resistant gene was inserted into tobacco, leading to the first genetically engineered plant. Advances followed that allowed scientists to manipulate and add genes to a variety of different organisms and induce a range of different effects. Plants were first commercialized with virus resistant tobacco released in China in 1992. The first genetically modified food was the Flavr Savr tomato marketed in 1994. By 2010, 29 countries had planted commercialized biotech crops. In 2000 a paper published in Science introduced golden rice, the first food developed with increased nutrient value.

The Séralini affair was the controversy surrounding the publication, retraction, and republication of a journal article by French molecular biologist Gilles-Éric Séralini. First published by Food and Chemical Toxicology in September 2012, the article presented a two-year feeding study in rats, and reported an increase in tumors among rats fed genetically modified corn and the herbicide RoundUp. Scientists and regulatory agencies subsequently concluded that the study's design was flawed and its findings unsubstantiated. A chief criticism was that each part of the study had too few rats to obtain statistically useful data, particularly because the strain of rat used, Sprague Dawley, develops tumors at a high rate over its lifetime.

The March Against Monsanto was an international grassroots movement and protest against Monsanto, a producer of genetically modified organisms (GMOs) and Roundup, a glyphosate-based herbicide. The movement was founded by Tami Canal in response to the failure of California Proposition 37, a ballot initiative which would have required labeling food products made from GMOs. Advocates support mandatory labeling laws for food made from GMOs.

GMO conspiracy theories are conspiracy theories related to the production and sale of genetically modified crops and genetically modified food. These conspiracy theories include claims that agribusinesses, especially Monsanto, have suppressed data showing that GMOs cause harm, deliberately cause food shortages to promote the use of GM food, or have co-opted government agencies such as the United States Food and Drug Administration or scientific societies such as the American Association for the Advancement of Science.
Chinmoy Sankar Dey is an Indian molecular biologist and a professor at Kusuma School of Biological Sciences of the Indian Institute of Technology, Delhi. Known for his research on insulin resistance, Dey's is a J. C. Bose National Fellow of the Department of Science and Technology and an elected fellow of the National Academy of Sciences, India and the Indian National Science Academy. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Medical Sciences in 2003. He is also a recipient of the National Bioscience Award for Career Development of the Department of Biotechnology.









