
Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
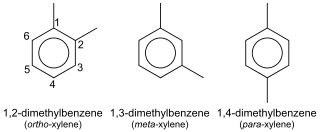
In organic chemistry, xylene or xylol are any of three organic compounds with the formula (CH3)2C6H4. They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value.

An oil refinery or petroleum refinery is an industrial process plant where petroleum is transformed and refined into useful products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petroleum gas and petroleum naphtha. Petrochemical feedstock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha. The crude oil feedstock has typically been processed by an oil production plant. There is usually an oil depot at or near an oil refinery for the storage of incoming crude oil feedstock as well as bulk liquid products. In 2020, the total capacity of global refineries for crude oil was about 101.2 million barrels per day.

In petrochemistry, petroleum geology and organic chemistry, cracking is the process whereby complex organic molecules such as kerogens or long-chain hydrocarbons are broken down into simpler molecules such as light hydrocarbons, by the breaking of carbon-carbon bonds in the precursors. The rate of cracking and the end products are strongly dependent on the temperature and presence of catalysts. Cracking is the breakdown of large hydrocarbons into smaller, more useful alkanes and alkenes. Simply put, hydrocarbon cracking is the process of breaking a long chain hydrocarbon into short ones. This process requires high temperatures.

Vladimir Nikolayevich Ipatieff, also Ipatyev was a Russian and American chemist. His most important contributions are in the field of petroleum chemistry and catalysts.
Butene, also known as butylene, is an alkene with the formula C4H8. The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products, and the butene is extracted from this by fractional distillation.

Catalytic reforming is a chemical process used to convert petroleum refinery naphthas distilled from crude oil into high-octane liquid products called reformates, which are premium blending stocks for high-octane gasoline. The process converts low-octane linear hydrocarbons (paraffins) into branched alkanes (isoparaffins) and cyclic naphthenes, which are then partially dehydrogenated to produce high-octane aromatic hydrocarbons. The dehydrogenation also produces significant amounts of byproduct hydrogen gas, which is fed into other refinery processes such as hydrocracking. A side reaction is hydrogenolysis, which produces light hydrocarbons of lower value, such as methane, ethane, propane and butanes.

p-Xylene (para-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The p- stands for para-, indicating that the two methyl groups in p-xylene occupy the diametrically opposite substituent positions 1 and 4. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and m-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable. The odor threshold of p-xylene is 0.62 parts per million (ppm).

m-Xylene (meta-xylene) is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The m- stands for meta-, indicating that the two methyl groups in m-xylene occupy positions 1 and 3 on a benzene ring. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and p-xylene. All have the same chemical formula C6H4(CH3)2. All xylene isomers are colorless and highly flammable.

Fluid Catalytic Cracking (FCC) is the conversion process used in petroleum refineries to convert the high-boiling point, high-molecular weight hydrocarbon fractions of petroleum into gasoline, alkene gases, and other petroleum products. The cracking of petroleum hydrocarbons was originally done by thermal cracking, now virtually replaced by catalytic cracking, which yields greater volumes of high octane rating gasoline; and produces by-product gases, with more carbon-carbon double bonds, that are of greater economic value than the gases produced by thermal cracking.

Hydrodesulfurization (HDS), also called hydrotreatment or hydrotreating, is a catalytic chemical process widely used to remove sulfur (S) from natural gas and from refined petroleum products, such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils. The purpose of removing the sulfur, and creating products such as ultra-low-sulfur diesel, is to reduce the sulfur dioxide emissions that result from using those fuels in automotive vehicles, aircraft, railroad locomotives, ships, gas or oil burning power plants, residential and industrial furnaces, and other forms of fuel combustion.

Visakhapatnam Refinery, is one of the two oil refineries of HPCL in India, the other being Mumbai Refinery. This was one of the first major industries of Visakhapatnam and first oil refinery on the East Coast. After the nationalisation, HPCL has transformed itself into a mega Public Sector Undertaking and it is second largest integrated oil company in India.

The Penex process is a continuous catalytic process used in the refining of crude oil. It isomerizes light naphtha (C5/C6) into higher-octane, branched C5/C6 molecules. It also reduces the concentration of benzene in the gasoline pool. It was first used commercially in 1958. Ideally, the isomerization catalyst converts normal pentane (nC5) to isopentane (iC5) and normal hexane (nC6) to 2,2- and 2,3-dimethylbutane. The thermodynamic equilibrium is more favorable at low temperature.

The Indian Institute of Petroleum (IIP), established in 1960, is one of the 37 constituent laboratories of the Council of Scientific and Industrial Research (CSIR), dedicated to R&D in the hydrocarbon sector.

Edith Marie Flanigen is a noted American chemist, known for her work on synthesis of emeralds, and later zeolites for molecular sieves at Union Carbide.

In the petroleum refining and petrochemical industries, the initialism BTX refers to mixtures of benzene, toluene, and the three xylene isomers, all of which are aromatic hydrocarbons. The xylene isomers are distinguished by the designations ortho –, meta –, and para – as indicated in the adjacent diagram. If ethylbenzene is included, the mixture is sometimes referred to as BTEX.
Gustav Egloff (1886–1955) was an American chemist nicknamed Gasoline Gus. He was Universal Oil Products' first chemist and by 1917 became their director, serving in that capacity until death. Science magazine described him as a "human catalyst".
Herman Pines was a Russian Empire-born American chemist. Born in Łódź—then part of the Russian Empire—he left his hometown as a young man as Jewish quotas and other anti-Jewish practices prevented Jewish students from attending university. After earning a degree in chemical engineering at the École Supérieure de Chimie Industrielle de Lyon in France, he worked at Universal Oil Products from 1930 to 1952. Pines also worked at Northwestern University beginning in 1941, and served from 1953–1970 as the Ipatieff Research Professor of Chemistry and director of the Ipatieff High Pressure and Catalytic Laboratory.
The Dangote Refinery is an oil refinery owned by Dangote Group that was inaugurated on the 22nd of May 2023 in Lekki, Nigeria. When in full operation, it is expected to have the capacity to process about 650,000 barrels per day of crude oil, making it the largest single-train refinery in the world. The investment is over 19 billion US dollars.

















