
Diabetic ketoacidosis (DKA) is a potentially life-threatening complication of diabetes mellitus. Signs and symptoms may include vomiting, abdominal pain, deep gasping breathing, increased urination, weakness, confusion and occasionally loss of consciousness. A person's breath may develop a specific "fruity" smell. The onset of symptoms is usually rapid. People without a previous diagnosis of diabetes may develop DKA as the first obvious symptom.

Pentamidine is an antimicrobial medication used to treat African trypanosomiasis, leishmaniasis, Balamuthia infections, babesiosis, and to prevent and treat pneumocystis pneumonia (PCP) in people with poor immune function. In African trypanosomiasis it is used for early disease before central nervous system involvement, as a second line option to suramin. It is an option for both visceral leishmaniasis and cutaneous leishmaniasis. Pentamidine can be given by injection into a vein or muscle or by inhalation.
Diuresis is the excretion of urine, especially when excessive (polyuria). The term collectively denotes the physiologic processes underpinning increased urine production by the kidneys during maintenance of fluid balance.

Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, acute mountain sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure and to alkalinize urine. It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out. It is taken by mouth or injection into a vein. Acetazolamide is a first generation carbonic anhydrase inhibitor and it decreases the ocular fluid and osmolality in the eye to decrease intraocular pressure.
Acidosis is a process causing increased acidity in the blood and other body tissues. If not further qualified, it usually refers to acidity of the blood plasma.
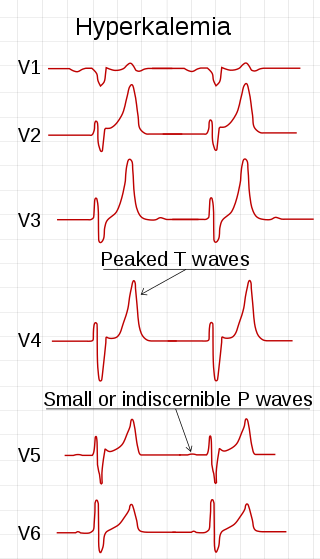
Hyperkalemia is an elevated level of potassium (K+) in the blood. Normal potassium levels are between 3.5 and 5.0 mmol/L (3.5 and 5.0 mEq/L) with levels above 5.5 mmol/L defined as hyperkalemia. Typically hyperkalemia does not cause symptoms. Occasionally when severe it can cause palpitations, muscle pain, muscle weakness, or numbness. Hyperkalemia can cause an abnormal heart rhythm which can result in cardiac arrest and death.

Electrolyte imbalance, or water-electrolyte imbalance, is an abnormality in the concentration of electrolytes in the body. Electrolytes play a vital role in maintaining homeostasis in the body. They help to regulate heart and neurological function, fluid balance, oxygen delivery, acid–base balance and much more. Electrolyte imbalances can develop by consuming too little or too much electrolyte as well as excreting too little or too much electrolyte. Examples of electrolytes include calcium, chloride, magnesium, phosphate, potassium, and sodium.
Hypernatremia, also spelled hypernatraemia, is a high concentration of sodium in the blood. Early symptoms may include a strong feeling of thirst, weakness, nausea, and loss of appetite. Severe symptoms include confusion, muscle twitching, and bleeding in or around the brain. Normal serum sodium levels are 135–145 mmol/L. Hypernatremia is generally defined as a serum sodium level of more than 145 mmol/L. Severe symptoms typically only occur when levels are above 160 mmol/L.
Hyperchloremia is an electrolyte disturbance in which there is an elevated level of chloride ions in the blood. The normal serum range for chloride is 96 to 106 mEq/L, therefore chloride levels at or above 110 mEq/L usually indicate kidney dysfunction as it is a regulator of chloride concentration. As of now there are no specific symptoms of hyperchloremia; however, it can be influenced by multiple abnormalities that cause a loss of electrolyte-free fluid, loss of hypotonic fluid, or increased administration of sodium chloride. These abnormalities are caused by diarrhea, vomiting, increased sodium chloride intake, renal dysfunction, diuretic use, and diabetes. Hyperchloremia should not be mistaken for hyperchloremic metabolic acidosis as hyperchloremic metabolic acidosis is characterized by two major changes: a decrease in blood pH and bicarbonate levels, as well as an increase in blood chloride levels. Instead those with hyperchloremic metabolic acidosis are usually predisposed to hyperchloremia.

Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.

Saline is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein, it is used to treat dehydration such as that from gastroenteritis and diabetic ketoacidosis. Large amounts may result in fluid overload, swelling, acidosis, and high blood sodium. In those with long-standing low blood sodium, excessive use may result in osmotic demyelination syndrome.

Ringer's lactate solution (RL), also known as sodium lactate solution,Lactated Ringer's, and Hartmann's solution, is a mixture of sodium chloride, sodium lactate, potassium chloride, and calcium chloride in water. It is used for replacing fluids and electrolytes in those who have low blood volume or low blood pressure. It may also be used to treat metabolic acidosis and to wash the eye following a chemical burn. It is given by intravenous infusion or applied to the affected area.

Metabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range (7.35–7.45). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. The condition typically cannot last long if the kidneys are functioning properly.
A basic metabolic panel (BMP) is a blood test consisting of a set of seven or eight biochemical tests and is one of the most common lab tests ordered by health care providers. Outside the United States, blood tests made up of the majority of the same biochemical tests are called urea and electrolytes, or urea, electrolytes, creatinine, and are often referred to as 'kidney function tests' as they also include a calculated estimated glomerular filtration rate. The BMP provides key information regarding fluid and electrolyte status, kidney function, blood sugar levels, and response to various medications and other medical therapies. It is frequently employed as a screening tool during a physical exam.

High anion gap metabolic acidosis is a form of metabolic acidosis characterized by a high anion gap. Metabolic acidosis occurs when the body produces too much acid, or when the kidneys are not removing enough acid from the body. Several types of metabolic acidosis occur, grouped by their influence on the anion gap.

Tricyclic antidepressant overdose is poisoning caused by excessive medication of the tricyclic antidepressant (TCA) type. Symptoms may include elevated body temperature, blurred vision, dilated pupils, sleepiness, confusion, seizures, rapid heart rate, and cardiac arrest. If symptoms have not occurred within six hours of exposure they are unlikely to occur.

Salicylate poisoning, also known as aspirin poisoning, is the acute or chronic poisoning with a salicylate such as aspirin. The classic symptoms are ringing in the ears, nausea, abdominal pain, and a fast breathing rate. Early on, these may be subtle, while larger doses may result in fever. Complications can include swelling of the brain or lungs, seizures, low blood sugar, or cardiac arrest.
The Hs and Ts is a mnemonic used to aid in remembering the possible reversible causes of cardiac arrest. A variety of disease processes can lead to a cardiac arrest; however, they usually boil down to one or more of the "Hs and Ts".
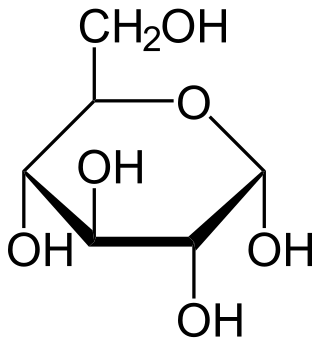
Intravenous sugar solution, also known as dextrose solution, is a mixture of dextrose (glucose) and water. It is used to treat low blood sugar or water loss without electrolyte loss. Water loss without electrolyte loss may occur in fever, hyperthyroidism, high blood calcium, or diabetes insipidus. It is also used in the treatment of high blood potassium, diabetic ketoacidosis, and as part of parenteral nutrition. It is given by injection into a vein.
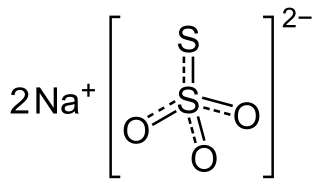
Sodium thiosulfate, also spelled sodium thiosulphate, is used as a medication to treat cyanide poisoning, pityriasis versicolor, and to decrease side effects from cisplatin. For cyanide poisoning, it is often used after the medication sodium nitrite and is typically only recommended for severe cases. It is either given by injection into a vein or applied to the skin.














