
A gametophyte is one of the two alternating multicellular phases in the life cycles of plants and algae. It is a haploid multicellular organism that develops from a haploid spore that has one set of chromosomes. The gametophyte is the sexual phase in the life cycle of plants and algae. It develops sex organs that produce gametes, haploid sex cells that participate in fertilization to form a diploid zygote which has a double set of chromosomes. Cell division of the zygote results in a new diploid multicellular organism, the second stage in the life cycle known as the sporophyte. The sporophyte can produce haploid spores by meiosis that on germination produce a new generation of gametophytes.

In biology, a spore is a unit of sexual or asexual reproduction that may be adapted for dispersal and for survival, often for extended periods of time, in unfavourable conditions. Spores form part of the life cycles of many plants, algae, fungi and protozoa. They were thought to have appeared as early as the mid-late Ordovician period as an adaptation of early land plants.

A sporangium ; pl.: sporangia) is an enclosure in which spores are formed. It can be composed of a single cell or can be multicellular. Virtually all plants, fungi, and many other groups form sporangia at some point in their life cycle. Sporangia can produce spores by mitosis, but in land plants and many fungi, sporangia produce genetically distinct haploid spores by meiosis.

Alternation of generations is the predominant type of life cycle in plants and algae. In plants both phases are multicellular: the haploid sexual phase – the gametophyte – alternates with a diploid asexual phase – the sporophyte.

Lycopodiopsida is a class of vascular plants also known as lycopods or lycophytes. Members of the class are also called clubmosses, firmosses, spikemosses and quillworts. They have dichotomously branching stems bearing simple leaves called microphylls and reproduce by means of spores borne in sporangia on the sides of the stems at the bases of the leaves. Although living species are small, during the Carboniferous, extinct tree-like forms (Lepidodendrales) formed huge forests that dominated the landscape and contributed to coal deposits.

A sporophyte is the diploid multicellular stage in the life cycle of a plant or alga which produces asexual spores. This stage alternates with a multicellular haploid gametophyte phase.

The order Salviniales is an order of ferns in the class Polypodiopsida.

Isoetes lacustris, the lake quillwort or Merlin's grass, is a boreal quillwort native on both sides of the northern Atlantic Ocean. Synonyms include Isoetes hieroglyphica.

Microspores are land plant spores that develop into male gametophytes, whereas megaspores develop into female gametophytes. The male gametophyte gives rise to sperm cells, which are used for fertilization of an egg cell to form a zygote. Megaspores are structures that are part of the alternation of generations in many seedless vascular cryptogams, all gymnosperms and all angiosperms. Plants with heterosporous life cycles using microspores and megaspores arose independently in several plant groups during the Devonian period. Microspores are haploid, and are produced from diploid microsporocytes by meiosis.

Megaspores, also called macrospores, are a type of spore that is present in heterosporous plants. These plants have two spore types, megaspores and microspores. Generally speaking, the megaspore, or large spore, germinates into a female gametophyte, which produces egg cells. These are fertilized by sperm produced by the male gametophyte developing from the microspore. Heterosporous plants include seed plants, water ferns (Salviniales), spikemosses (Selaginellaceae) and quillworts (Isoetaceae).

Isoetes engelmannii is a species of aquatic plant in the family Isoetaceae. It is referred to by the common names Engelmann's quillwort or Appalachian quillwort, and is the most widely distributed species of its genus in eastern North America. Its range extends from Ontario in the north, south to Florida and west Arkansas and Missouri. It can be found from April to October in temporary pools, bogs, marshes, stream edges, swamps and along wet roadsides.
Isoetes valida, commonly known as the strong quillwort or true quillwort, is an aquatic lycophyte native to eastern North America. It is found primarily in the Appalachian Mountains from Pennsylvania south to Alabama and Georgia. In addition, one collection of the plant was made in a railway ditch in Wilmington, Delaware in the 1860s, but this was most likely an accidental introduction.

Heterospory is the production of spores of two different sizes and sexes by the sporophytes of land plants. The smaller of these, the microspore, is male and the larger megaspore is female. Heterospory evolved during the Devonian period from isospory independently in several plant groups: the clubmosses, the ferns including the arborescent horsetails, and progymnosperms. This occurred as part of the process of evolution of the timing of sex differentiation.

Isoetes melanospora, commonly known as black-spored quillwort or black-spored Merlin's grass, is a rare and endangered aquatic lycophyte endemic to the U.S. states of Georgia and South Carolina.
Isoetes nuttallii, or Nuttall's quillwort, is a species of quillwort, a type of lycopod. It is native to shallow waters and other wet habitats of western North America from British Columbia to California. It produces up to 60 pointed, cylindrical, green to gray-green leaves, each 7 to 17 centimeters long. The velum completely covers the spherical sporangia, which are 5 millimeters long and 1.5 millimeters wide. The ligule is small and triangular. The megaspores are 400 to 500 micrometers in diameter. The microspores, which are spiny and covered in tubercles, are 28 to 31 micrometers long.

Isoetes echinospora, also known as spiny quillwort, spiny-spored quillwort or spring quillwort is a species of quillwort in the Isoetaceae family, and is the most abundant species in Canada. It can be found in shallow aquatic environments from Labrador and Newfoundland to Alaska, and south to Pennsylvania, Wisconsin, Michigan, Colorado, and California. In Germany it is found in only two locations: the Feldsee and Lake Titisee, both in the High Black Forest.

Isoetes riparia, the shore quillwort, is a species of plant in the family Isoetaceae. It can be found in rivers, creeks, and tidal mud flats in southern Quebec and southeastern Ontario, south to eastern New York. It has 5 to 35 long, erect bright green to yellow-green leaves, which are 6 to 35 centimeters long. The velum covers one fourth of the sporangium, which can be 7 millimeters long and 4 millimeters wide. The elongated ligule can grow to be 3 millimeters long. The spherical megaspores are 430 to 680 micrometers in diameter with closely set ridges. The kidney-shaped microspores are 24-35 micrometers long, and usually have spine-tipped tubercules. The megaspores can sometimes come to resemble that of either I. echinospora, if the megaspores become eroded and bear projections that could resemble spines, or I. macrospora, if the broken ridges take a certain shape.
Isoetes acadiensis, the Acadian quillwort is a species of quillwort in the Isoetaceae family described by Kott in 1981. It can be found along the shores of lakes, ponds, and rivers in Newfoundland, Nova Scotia, and New Brunswick, as well as in the American states Maine, Massachusetts, and New Hampshire. It has a similar distribution to that of I. tuckermanii. It bears 9 to 35 mostly recurved leaves, each 5–21 cm long. The leaves are usually dark green, though can occasionally be tinged with red. The sporangium can be up to five millimeters long and 3 millimeters in length, covered one sixth to one third by the velum. The spherical megaspores are 400-570 micrometers in diameter, and bear smooth ridges. The kidney shaped microspores are 25 to 30 micrometers long. It was originally believed to be a member of Isoetes hieroglyphica because of their similar megaspore structure.
Isoetes macrospora, the big-spore quillwort, is a species of quillwort in the Isoetaceae family. It can be found in the deep water of low nutrient lakes in the Precambrian Shield as well as in Newfoundland, Nova Scotia, Quebec, and Ontario. In the United States, it has been found in Minnesota and south, through the Appalachian Mountains to Virginia. It bears 3 to 17 long, stiff dark green leaves, sometimes with recurving tips. The sporangium can be 5 millimeters long and 4 millimeters wide, covered from one sixth to one quarter by the velum. The triangular ligule can grow up to 2 millimeters long. The spherical, white megaspores are 400 to 800 micrometers in diameter, and bear ridges that form honeycomb-like areas. The kidney-shaped microspores are 32 to 50 micrometers long, each with evenly spaced smooth papillae.
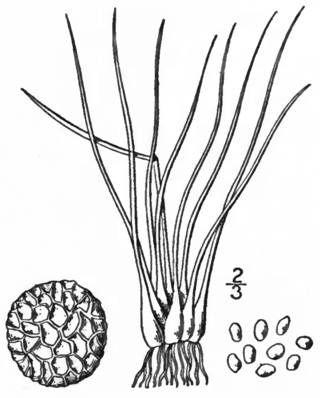
Isoetes tuckermanii, or Tuckerman's quillwort, is a tetraploid species of plant in the family Isoetaceae. It can be found in shallow water in Newfoundland, Nova Scotia, New Brunswick, and south through the New England states to Maryland. It bears 10 to 45 long bright green to yellow green leaves that are 4 to 25 centimeters long, usually erect, but sometimes recurved. The velum covers one fourth or less of the sporangium, which is usually unspotted, 5 millimeters long, and 3 millimeters wide. The white spherical megaspores are 400 to 650 micrometers in diameter, and bear rough-crested ridges that form a hexagonal honeycomb shape. The kidney shaped microspores are 24 to 33 micrometers long, bearing tubercles. It is very similar to I. macrospora, only reliably distinguishable by cytology or through careful megaspore measurement.

















