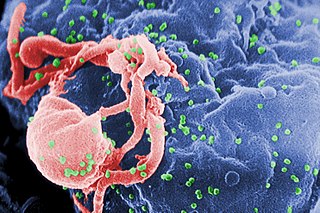
The human immunodeficiency viruses (HIV) are two species of Lentivirus that infect humans. Over time, they cause acquired immunodeficiency syndrome (AIDS), a condition in which progressive failure of the immune system allows life-threatening opportunistic infections and cancers to thrive. Without treatment, average survival time after infection with HIV is estimated to be 9 to 11 years, depending on the HIV subtype.
An inverted repeat is a single stranded sequence of nucleotides followed downstream by its reverse complement. The intervening sequence of nucleotides between the initial sequence and the reverse complement can be any length including zero. For example, 5'---TTACGnnnnnnCGTAA---3' is an inverted repeat sequence. When the intervening length is zero, the composite sequence is a palindromic sequence.

Picornaviruses are a group of related nonenveloped RNA viruses which infect vertebrates including fish, mammals, and birds. They are viruses that represent a large family of small, positive-sense, single-stranded RNA viruses with a 30 nm icosahedral capsid. The viruses in this family can cause a range of diseases including the common cold, poliomyelitis, meningitis, hepatitis, and paralysis.
VPg is a protein that is covalently attached to the 5′ end of positive strand viral RNA and acts as a primer during RNA synthesis in a variety of virus families including Picornaviridae, Potyviridae and Caliciviridae. There are some studies showing that a possible VPg protein is also present in astroviridae, however, experimental evidence for this has not yet been provided and requires further study. The primer activity of VPg occurs when the protein becomes uridylated, providing a free hydroxyl that can be extended by the virally encoded RNA-dependent RNA polymerase. For some virus families, VPg also has a role in translation initiation by acting like a 5' mRNA cap.
The genome and proteins of HIV have been the subject of extensive research since the discovery of the virus in 1983. "In the search for the causative agent, it was initially believed that the virus was a form of the Human T-cell leukemia virus (HTLV), which was known at the time to affect the human immune system and cause certain leukemias. However, researchers at the Pasteur Institute in Paris isolated a previously unknown and genetically distinct retrovirus in patients with AIDS which was later named HIV." Each virion comprises a viral envelope and associated matrix enclosing a capsid, which itself encloses two copies of the single-stranded RNA genome and several enzymes. The discovery of the virus itself occurred two years following the report of the first major cases of AIDS-associated illnesses.

A long terminal repeat (LTR) is a pair of identical sequences of DNA, several hundred base pairs long, which occur in eukaryotic genomes on either end of a series of genes or pseudogenes that form a retrotransposon or an endogenous retrovirus or a retroviral provirus. All retroviral genomes are flanked by LTRs, while there are some retrotransposons without LTRs. Typically, an element flanked by a pair of LTRs will encode a reverse transcriptase and an integrase, allowing the element to be copied and inserted at a different location of the genome. Copies of such an LTR-flanked element can often be found hundreds or thousands of times in a genome. LTR retrotransposons comprise about 8% of the human genome.

The hairpin ribozyme is a small section of RNA that can act as a ribozyme. Like the hammerhead ribozyme it is found in RNA satellites of plant viruses. It was first identified in the minus strand of the tobacco ringspot virus (TRSV) satellite RNA where it catalyzes self-cleavage and joining (ligation) reactions to process the products of rolling circle virus replication into linear and circular satellite RNA molecules. The hairpin ribozyme is similar to the hammerhead ribozyme in that it does not require a metal ion for the reaction.

The retroviral psi packaging element, also known as the Ψ RNA packaging signal, is a cis-acting RNA element identified in the genomes of the retroviruses Human immunodeficiency virus (HIV) and Simian immunodeficiency virus (SIV). It is involved in regulating the essential process of packaging the retroviral RNA genome into the viral capsid during replication. The final virion contains a dimer of two identical unspliced copies of the viral genome.
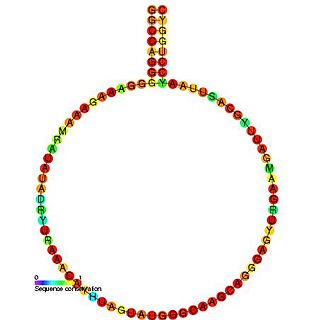
HIV gag stem loop 3 (GSL3) is a secondary structural component of the Retroviral Psi packaging element, also known as the psi recognition element. This domain plays a major role in RNA packaging and is located the 5’ untranslated region of the unspliced HIV-1 genome. GSL3 is known to direct specific packaging of HIV-1 genomic RNA. While deletion of GSL3 leads to decreases in both viral RNA packaging and dimerization, mutagenic studies have shown that it does not eliminate encapsulation of retroviral RNA.
The HIV-1 Rev response element (RRE) is a highly structured, ~350 nucleotide RNA segment present in the Env coding region of unspliced and partially spliced viral mRNAs. In the presence of the HIV-1 accessory protein Rev, HIV-1 mRNAs that contain the RRE can be exported from the nucleus to the cytoplasm for downstream events such as translation and virion packaging.

The HIV trans-activation response (TAR) element is an RNA element which is known to be required for the trans-activation of the viral promoter and for virus replication. The TAR hairpin is a dynamic structure that acts as a binding site for the Tat protein, and this interaction stimulates the activity of the long terminal repeat promoter.

HIV-1 protease (PR) is a retroviral aspartyl protease (retropepsin), an enzyme involved with peptide bond hydrolysis in retroviruses, that is essential for the life-cycle of HIV, the retrovirus that causes AIDS. HIV protease cleaves newly synthesized polyproteins at nine cleavage sites to create the mature protein components of an HIV virion, the infectious form of a virus outside of the host cell. Without effective HIV protease, HIV virions remain uninfectious.

HIV Tat-specific factor 1 is a protein that in humans is encoded by the HTATSF1 gene.

RISC-loading complex subunit TARBP2 is a protein that in humans is encoded by the TARBP2 gene.

TATA-binding protein-associated factor 172 is a protein that in humans is encoded by the BTAF1 gene.
General transcription factor IIF subunit 2 is a protein that in humans is encoded by the GTF2F2 gene.

Probable methyltransferase TARBP1 is an enzyme that in humans is encoded by the TARBP1 gene.

In molecular biology, Tat is a protein that is encoded for by the tat gene in HIV-1. Tat is a regulatory protein that drastically enhances the efficiency of viral transcription. Tat stands for "Trans-Activator of Transcription". The protein consists of between 86 and 101 amino acids depending on the subtype. Tat vastly increases the level of transcription of the HIV dsDNA. Before Tat is present, a small number of RNA transcripts will be made, which allow the Tat protein to be produced. Tat then binds to cellular factors and mediates their phosphorylation, resulting in increased transcription of all HIV genes, providing a positive feedback cycle. This in turn allows HIV to have an explosive response once a threshold amount of Tat is produced, a useful tool for defeating the body's response.

Rev is a transactivating protein that is essential to the regulation of HIV-1 protein expression. A nuclear localization signal is encoded in the rev gene, which allows the Rev protein to be localized to the nucleus, where it is involved in the export of unspliced and incompletely spliced mRNAs. In the absence of Rev, mRNAs of the HIV-1 late (structural) genes are retained in the nucleus, preventing their translation.
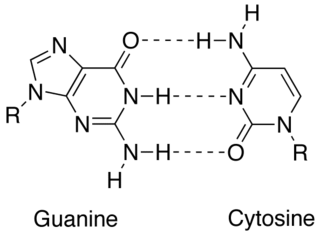
In molecular biology, complementarity describes a relationship between two structures each following the lock-and-key principle. In nature complementarity is the base principle of DNA replication and transcription as it is a property shared between two DNA or RNA sequences, such that when they are aligned antiparallel to each other, the nucleotide bases at each position in the sequences will be complementary, much like looking in the mirror and seeing the reverse of things. This complementary base pairing allows cells to copy information from one generation to another and even find and repair damage to the information stored in the sequences.

















