Related Research Articles

Organic electronics is a field of materials science concerning the design, synthesis, characterization, and application of organic molecules or polymers that show desirable electronic properties such as conductivity. Unlike conventional inorganic conductors and semiconductors, organic electronic materials are constructed from organic (carbon-based) molecules or polymers using synthetic strategies developed in the context of organic chemistry and polymer chemistry.

An organic light-emitting diode (OLED), also known as organic electroluminescentdiode, is a type of light-emitting diode (LED) in which the emissive electroluminescent layer is an organic compound film that emits light in response to an electric current. This organic layer is situated between two electrodes; typically, at least one of these electrodes is transparent. OLEDs are used to create digital displays in devices such as television screens, computer monitors, and portable systems such as smartphones and handheld game consoles. A major area of research is the development of white OLED devices for use in solid-state lighting applications.

Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic in nanotechnology and materials science. When a quantum dot is illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conductance band. The excited electron can drop back into the valence band releasing its energy as light. This light emission (photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the conductance band and the valence band, or the transition between discrete energy states when the band structure is no longer well-defined in QDs.

Conductive polymers or, more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The main advantage of conductive polymers is that they are easy to process, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermoformable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.

Poly(p-phenylene vinylene) (PPV, or polyphenylene vinylene) is a conducting polymer of the rigid-rod polymer family. PPV is the only polymer of this type that can be processed into a highly ordered crystalline thin film. PPV and its derivatives are electrically conducting upon doping. Although insoluble in water, its precursors can be manipulated in aqueous solution. The small optical band gap and its bright yellow fluorescence makes PPV a candidate in applications such as light-emitting diodes (LED) and photovoltaic devices. Moreover, PPV can be doped to form electrically conductive materials. Its physical and electronic properties can be altered by the inclusion of functional side groups.
Organic semiconductors are solids whose building blocks are pi-bonded molecules or polymers made up by carbon and hydrogen atoms and – at times – heteroatoms such as nitrogen, sulfur and oxygen. They exist in the form of molecular crystals or amorphous thin films. In general, they are electrical insulators, but become semiconducting when charges are either injected from appropriate electrodes, upon doping or by photoexcitation.

A flexible organic light-emitting diode (FOLED) is a type of organic light-emitting diode (OLED) incorporating a flexible plastic substrate on which the electroluminescent organic semiconductor is deposited. This enables the device to be bent or rolled while still operating. Currently the focus of research in industrial and academic groups, flexible OLEDs form one method of fabricating a rollable display.
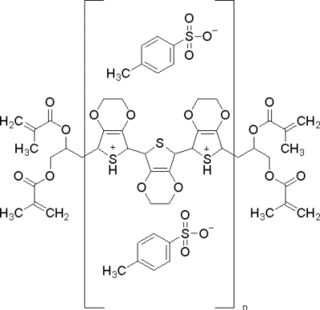
Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) is a polymer mixture of two ionomers. One component in this mixture is made up of polystyrene sulfonate which is a sulfonated polystyrene. Part of the sulfonyl groups are deprotonated and carry a negative charge. The other component poly(3,4-ethylenedioxythiophene) (PEDOT) is a conjugated polymer and carries positive charges and is based on polythiophene. Together the charged macromolecules form a macromolecular salt.
Quantum-cascade lasers (QCLs) are semiconductor lasers that emit in the mid- to far-infrared portion of the electromagnetic spectrum and were first demonstrated by Jérôme Faist, Federico Capasso, Deborah Sivco, Carlo Sirtori, Albert Hutchinson, and Alfred Cho at Bell Laboratories in 1994.
Phosphorescent organic light-emitting diodes (PHOLED) are a type of organic light-emitting diode (OLED) that use the principle of phosphorescence to obtain higher internal efficiencies than fluorescent OLEDs. This technology is currently under development by many industrial and academic research groups.
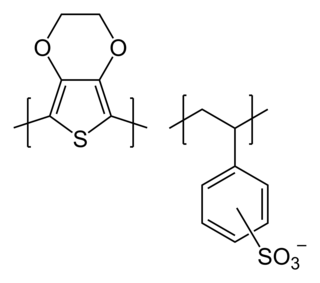
Poly(3,4-ethylenedioxythiophene)-tetramethacrylate or PEDOT-TMA is a p-type conducting polymer based on 3,4-ethylenedioxylthiophene or the EDOT monomer. It is a modification of the PEDOT structure. Advantages of this polymer relative to PEDOT are that it is dispersible in organic solvents, and it is non-corrosive. PEDOT-TMA was developed under a contract with the National Science Foundation, and it was first announced publicly on April 12, 2004. The trade name for PEDOT-TMA is Oligotron. PEDOT-TMA was featured in an article entitled "Next Stretch for Plastic Electronics" that appeared in Scientific American in 2004. The U.S. Patent office issued a patent protecting PEDOT-TMA on April 22, 2008.
Organic photovoltaic devices (OPVs) are fabricated from thin films of organic semiconductors, such as polymers and small-molecule compounds, and are typically on the order of 100 nm thick. Because polymer based OPVs can be made using a coating process such as spin coating or inkjet printing, they are an attractive option for inexpensively covering large areas as well as flexible plastic surfaces. A promising low cost alternative to conventional solar cells made of crystalline silicon, there is a large amount of research being dedicated throughout industry and academia towards developing OPVs and increasing their power conversion efficiency.
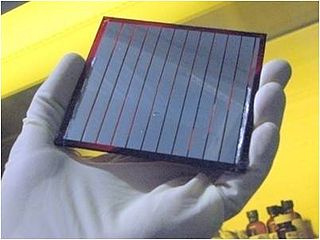
An organic solar cell (OSC) or plastic solar cell is a type of photovoltaic that uses organic electronics, a branch of electronics that deals with conductive organic polymers or small organic molecules, for light absorption and charge transport to produce electricity from sunlight by the photovoltaic effect. Most organic photovoltaic cells are polymer solar cells.

Steven Van Slyke is an American chemist, best known for his co-invention of the Organic Light Emitting Diode (OLED) with Ching Wan Tang and his contributions to the commercial development of OLED displays. Van Slyke is currently the Chief Technology Officer Emeritus at Kateeva, Inc. Prior to joining Kateeva, he held various positions at Eastman Kodak and was involved in all aspects of OLED technology, from basic materials development to implementation of full-color OLED display manufacturing.

A quantum dot display is a display device that uses quantum dots (QD), semiconductor nanocrystals which can produce pure monochromatic red, green, and blue light. Photo-emissive quantum dot particles are used in LCD backlights or display color filters. Quantum dots are excited by the blue light from the display panel to emit pure basic colors, which reduces light losses and color crosstalk in color filters, improving display brightness and color gamut. Light travels through QD layer film and traditional RGB filters made from color pigments, or through QD filters with red/green QD color converters and blue passthrough. Although the QD color filter technology is primarily used in LED-backlit LCDs, it is applicable to other display technologies which use color filters, such as blue/UV active-matrix organic light-emitting diode (AMOLED) or QNED/MicroLED display panels. LED-backlit LCDs are the main application of photo-emissive quantum dots, though blue organic light-emitting diode (OLED) panels with QD color filters are being researched.

Polyfluorene is a polymer with formula (C13H8)n, consisting of fluorene units linked in a linear chain — specifically, at carbon atoms 2 and 7 in the standard fluorene numbering. It can also be described as a chain of benzene rings linked in para positions with an extra methylene bridge connecting every pair of rings.
A liquid-crystal laser is a laser that uses a liquid crystal as the resonator cavity, allowing selection of emission wavelength and polarization from the active laser medium. The lasing medium is usually a dye doped into the liquid crystal. Liquid-crystal lasers are comparable in size to diode lasers, but provide the continuous wide spectrum tunability of dye lasers while maintaining a large coherence area. The tuning range is typically several tens of nanometers. Self-organization at micrometer scales reduces manufacturing complexity compared to using layered photonic metamaterials. Operation may be either in continuous wave mode or in pulsed mode.
The organic electrochemical transistor (OECT) is an organic electronic device which functions like a transistor. The current flowing through the device is controlled by the exchange of ions between an electrolyte and the OECT channel composed of an organic conductor or semiconductor. The exchange of ions is driven by a voltage applied to the gate electrode which is in ionic contact with the channel through the electrolyte. The migration of ions between the channel and the electrolyte is accompanied by electrochemical redox reactions occurring in the channel material. The electrochemical redox of the channel along with ion migration changes the conductivity of the channel in a process called electrochemical doping. OECTs are being explored for applications in biosensors, bioelectronics and large-area, low-cost electronics. OECTs can also be used as multi-bit memory devices that mimic the synaptic functionalities of the brain. For this reason, OECTs can be also being investigated as elements in neuromorphic computing applications.
Optoelectronic reciprocity relations relate properties of a diode under illumination to the photon emission of the same diode under applied voltage. The relations are useful for interpretation of luminescence based measurements of solar cells and modules and for the analysis of recombination losses in solar cells.
Light-emitting diodes (LEDs) produce light by the recombination of electrons and electron holes in a semiconductor, a process called "electroluminescence". The wavelength of the light produced depends on the energy band gap of the semiconductors used. Since these materials have a high index of refraction, design features of the devices such as special optical coatings and die shape are required to efficiently emit light. A LED is a long-lived light source, but certain mechanisms can cause slow loss of efficiency of the device or sudden failure. The wavelength of the light emitted is a function of the band gap of the semiconductor material used; materials such as gallium arsenide, and others, with various trace doping elements, are used to produce different colors of light. Another type of LED uses a quantum dot which can have its properties and wavelength adjusted by its size. Light-emitting diodes are widely used in indicator and display functions, and white LEDs are displacing other technologies for general illumination purposes.
References
- ↑ Gao, J.; Dane, J. (2003). "Planar Polymer Light-Emitting Electrochemical Cells with extremely Large Interelectrode Spacing". Applied Physics Letters. 83 (15): 3027. Bibcode:2003ApPhL..83.3027G. doi:10.1063/1.1618948.
- ↑ Shin, J.-H.; Dzwilewski, A.; Iwasiewicz, A.; Xiao, S.; Fransson, Å.; Ankah, G. N.; Edman, L. (2006). "Light Emission at 5 V from a Polymer Device with a Millimeter-Sized Interelectrode Gap". Applied Physics Letters. 89 (1): 013509. Bibcode:2006ApPhL..89a3509S. doi:10.1063/1.2219122.
- ↑ Matyba, P.; Yamaguchi, H.; Eda, G.; Chhowalla, M.; Edman, L.; Robinson, N. D. (2010). "Graphene and Mobile Ions: The Key to All-Plastic, Solution-Processed Light-Emitting Devices". ACS Nano. 4 (2): 637–42. CiteSeerX 10.1.1.474.2436 . doi:10.1021/nn9018569. PMID 20131906.
- ↑ Yu, Z.; Hu, L.; Liu, Z.; Sun, M.; Wang, M.; Grüner, G.; Pei, Q. (2009). "Fully Bendable Polymer Light Emitting Devices with Carbon Nanotubes as Cathode and Anode". Applied Physics Letters. 95 (20): 203304. Bibcode:2009ApPhL..95t3304Y. doi:10.1063/1.3266869.
- ↑ Mauthner, G.; Landfester, K.; Kock, A.; Bruckl, H.; Kast, M.; Stepper, C.; List, E. J. W. (2008). "Inkjet Printed Surface Cell Light-Emitting Devices from a Water-Based Polymer Dispersion". Organic Electronics. 9 (2): 164–70. doi:10.1016/j.orgel.2007.10.007.
- ↑ Gao, J.; Dane, J. (2004). "Visualization of Electrochemical Doping and Light-Emitting Junction Formation in Conjugated Polymer Films". Applied Physics Letters. 84 (15): 2778. Bibcode:2004ApPhL..84.2778G. doi: 10.1063/1.1702126 .
- ↑ Tang, Shi; Edman, Ludvig (2016-06-13). "Light-Emitting Electrochemical Cells: A Review on Recent Progress". Topics in Current Chemistry. 374 (4): 40. doi:10.1007/s41061-016-0040-4. ISSN 2365-0869. PMID 27573392. S2CID 5205115.
- ↑ Pei, Q. B.; Yu, G.; Zhang, C.; Yang, Y.; Heeger, A. J. (1995). "Polymer Light-Emitting Electrochemical-Cells". Science. 269 (5227): 1086–8. Bibcode:1995Sci...269.1086P. doi:10.1126/science.269.5227.1086. PMID 17755530. S2CID 36807816.
- ↑ Filiatrault, H. L.; Porteous, G. C.; Carmichael, R. S.; Davidson, G. J. E.; Carmichael, T. B. (2012). "Stretchable Light-Emitting Electrochemical Cells Using an Elastomeric Emissive Material". Advanced Materials. 24 (20): 2673–8. Bibcode:2012AdM....24.2673F. doi:10.1002/adma.201200448. PMID 22451224. S2CID 13047158.
- ↑ Sandström, A.; Dam, H. F.; Krebs, F. C.; Edman, L. (2012). "Ambient Fabrication of Flexible and Large-Area Organic Light-Emitting Devices Using Slot-Die Coating". Nature Communications. 3: 1002. Bibcode:2012NatCo...3.1002S. doi:10.1038/ncomms2002. PMC 3432459 . PMID 22893126.
- ↑ Tang, S.; Sandström, A.; Lundberg P.; Lanz, T.; Larsen, C.; van Reenen, S.; Kemerink, M.; Edman, L. (30 October 2017). "Design rules for light-emitting electrochemical cells delivering bright luminance at 27.5 percent external quantum efficiency". Nature Communications. 8 (1190 (2017)): 1190. Bibcode:2017NatCo...8.1190T. doi:10.1038/s41467-017-01339-0. PMC 5662711 . PMID 29085078.