Related Research Articles
Free-running sleep is a rare sleep pattern whereby the sleep schedule of a person shifts later every day. It occurs as the sleep disorder non-24-hour sleep–wake disorder or artificially as part of experiments used in the study of circadian and other rhythms in biology. Study subjects are shielded from all time cues, often by a constant light protocol, by a constant dark protocol or by the use of light/dark conditions to which the organism cannot entrain such as the ultrashort protocol of one hour dark and two hours light. Also, limited amounts of food may be made available at short intervals so as to avoid entrainment to mealtimes. Subjects are thus forced to live by their internal circadian "clocks".

A circadian rhythm, or circadian cycle, is a natural oscillation that repeats roughly every 24 hours. Circadian rhythms can refer to any process that originates within an organism and responds to the environment. Circadian rhythms are regulated by a circadian clock whose primary function is to rhythmically co-ordinate biological processes so they occur at the correct time to maximise the fitness of an individual. Circadian rhythms have been widely observed in animals, plants, fungi and cyanobacteria and there is evidence that they evolved independently in each of these kingdoms of life.

Chronobiology is a field of biology that examines timing processes, including periodic (cyclic) phenomena in living organisms, such as their adaptation to solar- and lunar-related rhythms. These cycles are known as biological rhythms. Chronobiology comes from the ancient Greek χρόνος, and biology, which pertains to the study, or science, of life. The related terms chronomics and chronome have been used in some cases to describe either the molecular mechanisms involved in chronobiological phenomena or the more quantitative aspects of chronobiology, particularly where comparison of cycles between organisms is required.

Delayed sleep phase disorder (DSPD), more often known as delayed sleep phase syndrome and also as delayed sleep–wake phase disorder, is the delaying of a person's circadian rhythm compared to those of societal norms. The disorder affects the timing of biological rhythms including sleep, peak period of alertness, core body temperature, and hormonal cycles.

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.
Non-24-hour sleep–wake disorder is one of several chronic circadian rhythm sleep disorders (CRSDs). It is defined as a "chronic steady pattern comprising [...] daily delays in sleep onset and wake times in an individual living in a society". Symptoms result when the non-entrained (free-running) endogenous circadian rhythm drifts out of alignment with the light–dark cycle in nature. Although this sleep disorder is more common in blind people, affecting up to 70% of the totally blind, it can also affect sighted people. Non-24 may also be comorbid with bipolar disorder, depression, and traumatic brain injury. The American Academy of Sleep Medicine (AASM) has provided CRSD guidelines since 2007 with the latest update released in 2015.
A phase response curve (PRC) illustrates the transient change in the cycle period of an oscillation induced by a perturbation as a function of the phase at which it is received. PRCs are used in various fields; examples of biological oscillations are the heartbeat, circadian rhythms, and the regular, repetitive firing observed in some neurons in the absence of noise.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of an additional photoreceptor was first suspected in 1927 when mice lacking rods and cones still responded to changing light levels through pupil constriction; this suggested that rods and cones are not the only light-sensitive tissue. However, it was unclear whether this light sensitivity arose from an additional retinal photoreceptor or elsewhere in the body. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore, they constitute a third class of photoreceptors, in addition to rod and cone cells.

High-energy visible light is short-wave light in the violet/blue band from 400 to 450 nm in the visible spectrum, which has a number of purported negative biological effects, namely on circadian rhythm and retinal health, which can lead to age-related macular degeneration. Increasingly, blue blocking filters are being designed into glasses to avoid blue light's purported negative effects. However, there is no good evidence that filtering blue light with spectacles has any effect on eye health, eye strain, sleep quality or vision quality.
Circadian rhythm sleep disorders (CRSD), also known as circadian rhythm sleep-wake disorders (CRSWD), are a family of sleep disorders which affect the timing of sleep. CRSDs arise from a persistent pattern of sleep/wake disturbances that can be caused either by dysfunction in one's biological clock system, or by misalignment between one's endogenous oscillator and externally imposed cues. As a result of this mismatch, those affected by circadian rhythm sleep disorders have a tendency to fall asleep at unconventional time points in the day. These occurrences often lead to recurring instances of disturbed rest, where individuals affected by the disorder are unable to go to sleep and awaken at "normal" times for work, school, and other social obligations. Delayed sleep phase disorder, advanced sleep phase disorder, non-24-hour sleep–wake disorder and irregular sleep–wake rhythm disorder represents the four main types of CRSD.
Melatonin receptors are G protein-coupled receptors (GPCR) which bind melatonin. Three types of melatonin receptors have been cloned. The MT1 (or Mel1A or MTNR1A) and MT2 (or Mel1B or MTNR1B) receptor subtypes are present in humans and other mammals, while an additional melatonin receptor subtype MT3 (or Mel1C or MTNR1C) has been identified in amphibia and birds. The receptors are crucial in the signal cascade of melatonin. In the field of chronobiology, melatonin has been found to be a key player in the synchrony of biological clocks. Melatonin secretion by the pineal gland has circadian rhythmicity regulated by the suprachiasmatic nucleus (SCN) found in the brain. The SCN functions as the timing regulator for melatonin; melatonin then follows a feedback loop to decrease SCN neuronal firing. The receptors MT1 and MT2 control this process. Melatonin receptors are found throughout the body in places such as the brain, the retina of the eye, the cardiovascular system, the liver and gallbladder, the colon, the skin, the kidneys, and many others. In 2019, X-ray crystal and cryo-EM structures of MT1 and MT2 were reported.
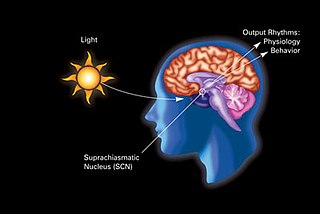
In neuroanatomy, the retinohypothalamic tract (RHT) is a photic neural input pathway involved in the circadian rhythms of mammals. The origin of the retinohypothalamic tract is the intrinsically photosensitive retinal ganglion cells (ipRGC), which contain the photopigment melanopsin. The axons of the ipRGCs belonging to the retinohypothalamic tract project directly, monosynaptically, to the suprachiasmatic nuclei (SCN) via the optic nerve and the optic chiasm. The suprachiasmatic nuclei receive and interpret information on environmental light, dark and day length, important in the entrainment of the "body clock". They can coordinate peripheral "clocks" and direct the pineal gland to secrete the hormone melatonin.
Ignacio Provencio is an American neuroscientist and the discoverer of melanopsin, an opsin found in specialized photosensitive ganglion cells of the mammalian retina. Provencio served as the program committee chair of the Society for Research on Biological Rhythms from 2008 to 2010.

Light in school buildings traditionally is from a combination of daylight and electric light to illuminate learning spaces, hallways, cafeterias, offices and other interior areas. Light fixtures currently in use usually provide students and teachers with satisfactory visual performance, i.e., the ability to read a book, have lunch, or play basketball in a gymnasium. However, classroom lighting may also affect students' circadian systems, which may in turn affect test scores, attendance and behavior.
Designing lighting for the elderly requires special consideration and care from architects and lighting designers. As people age, they experience neurodegeneration in the retina and in the suprachiasmatic nucleus (SCN). Less light reaches the back of the eyes because the pupils decrease in size as one ages, the lens inside one's eye becomes thicker, and the lens scatters more light, causing objects and colors to appear less vivid. These symptoms are particularly common with persons having alzheimer's disease. Older people also have reduced levels of retinal illuminance, such as having smaller pupils and less transparent crystalline lenses. Furthermore, as an individual ages, they begins to lose retinal neurons, which not only compromises the ability to see but also to register a robust daily pattern of light-dark that is needed to maintain biological rhythms. The 24-hour light-dark cycle is the most important external stimulus for regulating the timing of the circadian cycle.

Charles Andrew Czeisler is a Hungarian-American physician and sleep and circadian researcher. He is a leading researcher and author in the fields of the effects of light on human physiology, circadian rhythms and sleep medicine.
Johanna H. Meijer is a Dutch scientist who has contributed significantly to the field of chronobiology. Meijer has made notable contributions to the understanding of the neural and molecular mechanisms of circadian pacemakers. She is known for her extensive studies of photic and non-photic effects on the mammalian circadian clocks. Notably, Meijer is the 2016 recipient of the Aschoff and Honma Prize, one of the most prestigious international prizes in the circadian research field. In addition to still unraveling neuronal mechanisms of circadian clocks and their applications to health, Meijer's lab now studies the effects of modern lifestyles on our circadian rhythm and bodily functions.
Dr. Debra J. Skene is a chronobiologist with specific interest in the mammalian circadian rhythm and the consequences of disturbing the circadian system. She is also interested in finding their potential treatments for people who suffer from circadian misalignment. Skene and her team of researchers tackle these questions using animal models, clinical trials, and most recently, liquid chromatography-mass spectrometry. Most notably, Skene is credited for her evidence of a novel photopigment in humans, later discovered to be melanopsin. She was also involved in discovering links between human PER3 genotype and an extremely shifted sleep schedules categorized as extreme diurnal preference. Skene received her Bachelor of Pharmacy, Master of Science, and Ph.D. in South Africa.
In chronobiology, photoentrainment refers to the process by which an organism's biological clock, or circadian rhythm, synchronizes to daily cycles of light and dark in the environment. The mechanisms of photoentrainment differ from organism to organism. Photoentrainment plays a major role in maintaining proper timing of physiological processes and coordinating behavior within the natural environment. Studying organisms’ different photoentrainment mechanisms sheds light on how organisms may adapt to anthropogenic changes to the environment.
Elizabeth Klerman is a professor of neurology at Harvard Medical School. Her research focuses on applying circadian and sleep research principles to human physiology and pathophysiology. She also uses mathematical analysis and modeling to study human circadian, sleep, and objective neurobehavioral performance and subjective (self-reported) mood and alertness rhythms.
References
- ↑ Kolmos E, Davis SJ (September 2007). "Circadian rhythms: rho-related signals in time-specific light perception". cooment. Current Biology. 17 (18): R808–10. doi:10.1016/j.cub.2007.07.031. hdl: 11858/00-001M-0000-0012-3809-B . PMID 17878051. S2CID 10799409.
- 1 2 3 4 5 Duffy JF, Czeisler CA (June 2009). "Effect of Light on Human Circadian Physiology". review. Sleep Medicine Clinics. 4 (2): 165–177. doi:10.1016/j.jsmc.2009.01.004. PMC 2717723 . PMID 20161220.
- 1 2 3 4 5 Vimal RL, Pandey-Vimal MU, Vimal LS, Frederick BB, Stopa EG, Renshaw PF, et al. (January 2009). "Activation of suprachiasmatic nuclei and primary visual cortex depends upon time of day". primary. The European Journal of Neuroscience. 29 (2): 399–410. doi:10.1111/j.1460-9568.2008.06582.x. PMID 19200242. S2CID 41456654.
- 1 2 3 4 5 6 7 8 9 10 11 12 LeGates TA, Fernandez DC, Hattar S (July 2014). "Light as a central modulator of circadian rhythms, sleep and affect". review. Nature Reviews. Neuroscience. 15 (7): 443–54. doi:10.1038/nrn3743. PMC 4254760 . PMID 24917305.
- 1 2 3 4 Dijk DJ, Archer SN (June 2009). "Light, sleep, and circadian rhythms: together again". primary. PLOS Biology. 7 (6): e1000145. doi: 10.1371/journal.pbio.1000145 . PMC 2691600 . PMID 19547745.
- 1 2 3 4 5 6 7 Stephenson KM, Schroder CM, Bertschy G, Bourgin P (October 2012). "Complex interaction of circadian and non-circadian effects of light on mood: shedding new light on an old story". review. Sleep Medicine Reviews. 16 (5): 445–54. doi:10.1016/j.smrv.2011.09.002. PMID 22244990.
- 1 2 3 4 5 Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, et al. (January 2014). "Measuring and using light in the melanopsin age". review. Trends in Neurosciences. 37 (1): 1–9. doi:10.1016/j.tins.2013.10.004. PMC 4699304 . PMID 24287308.
- 1 2 3 4 5 Yan L (December 2009). "Expression of clock genes in the suprachiasmatic nucleus: effect of environmental lighting conditions". review. Reviews in Endocrine & Metabolic Disorders. 10 (4): 301–10. doi:10.1007/s11154-009-9121-9. PMID 19777352. S2CID 8653740.
- 1 2 van Bommel WJ (July 2006). "Non-visual biological effect of lighting and the practical meaning for lighting for work". review. Applied Ergonomics. 37 (4): 461–6. doi:10.1016/j.apergo.2006.04.009. PMID 16756935.
- ↑ Blume, Christine; Garbazza, Corrado; Spitschan, Manuel (2019). "Effects of light on human circadian rhythms, sleep and mood". Somnologie. 23 (3): 147–156. doi:10.1007/s11818-019-00215-x. ISSN 1432-9123. PMC 6751071 . PMID 31534436.
- 1 2 Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ (May 2003). "Phase advancing human circadian rhythms with short wavelength light". primary. Neuroscience Letters. 342 (1–2): 37–40. doi:10.1016/S0304-3940(03)00223-4. PMID 12727312. S2CID 913608.
- 1 2 3 Duffy JF, Kronauer RE, Czeisler CA (August 1996). "Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure". primary. The Journal of Physiology. 495 (Pt 1) (Pt 1): 289–97. doi:10.1113/jphysiol.1996.sp021593. PMC 1160744 . PMID 8866371.
- 1 2 Baehr EK, Fogg LF, Eastman CI (December 1999). "Intermittent bright light and exercise to entrain human circadian rhythms to night work". primary. The American Journal of Physiology. 277 (6): R1598–604. doi:10.1152/ajpregu.1999.277.6.R1598. PMID 10600904.
- ↑ Ma WP, Cao J, Tian M, Cui MH, Han HL, Yang YX, Xu L (October 2007). "Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats". primary. Neuroscience Research. 59 (2): 224–30. doi:10.1016/j.neures.2007.06.1474. PMID 17692419. S2CID 46433973.
- ↑ Gorman MR, Kendall M, Elliott JA (February 2005). "Scotopic illumination enhances entrainment of circadian rhythms to lengthening light:dark cycles". primary. Journal of Biological Rhythms. 20 (1): 38–48. doi: 10.1177/0748730404271573 . PMID 15654069. S2CID 35736954.
- ↑ Cajochen, Christian; Münch, Mirjam; Kobialka, Szymon; Kräuchi, Kurt; Steiner, Roland; Oelhafen, Peter; Orgül, Selim; Wirz-Justice, Anna (March 2005). "High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light". The Journal of Clinical Endocrinology and Metabolism. 90 (3): 1311–1316. doi: 10.1210/jc.2004-0957 . ISSN 0021-972X. PMID 15585546.
- ↑ Küller R, Wetterberg L (June 1993). "Melatonin, Cortisol, EEG, ECG and subjective comfort in healthy humans: Impact of two fluorescent lamp types at two light intensities". primary. Journal of Lighting Research and Technology. 25 (2): 71–80. doi:10.1177/096032719302500203. S2CID 143924924.
- 1 2 Bellia L, Bisegna F, Spada G (October 2011). "Lighting in indoor environments: Visual and non-visual effects of light sources with different spectral power distributions". primary. Building and Environment. 46 (10): 1984–92. doi:10.1016/j.buildenv.2011.04.007.
- 1 2 Vandewalle G, Gais S, Schabus M, Balteau E, Carrier J, Darsaud A, Sterpenich V, Albouy G, Dijk DJ, Maquet P (December 2007). "Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure". primary. Cerebral Cortex. 17 (12): 2788–95. doi: 10.1093/cercor/bhm007 . PMID 17404390.
- 1 2 Kubatka P, Zubor P, Busselberg D, Kwon TK, Adamek M, Petrovic D, et al. (February 2018). "Melatonin and breast cancer: Evidences from preclinical and human studies". review. Critical Reviews in Oncology/Hematology. 122: 133–143. doi:10.1016/j.critrevonc.2017.12.018. PMID 29458781.
- ↑ Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC (July 2002). "Light during darkness, melatonin suppression and cancer progression". review. Neuro Endocrinology Letters. 23 (Suppl 2): 52–6. PMID 12163849.
- ↑ van Someren EJ, Mirmiran M, Swaab DF (November 1993). "Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer's disease: chronobiological perspectives". review. Behavioural Brain Research. 57 (2): 235–53. doi:10.1016/0166-4328(93)90140-L. hdl: 20.500.11755/2b612915-d99b-4824-a0a3-cfe17279393b . PMID 8117428. S2CID 4015259.
- ↑ Vandewalle G, Balteau E, Phillips C, Degueldre C, Moreau V, Sterpenich V, et al. (August 2006). "Daytime light exposure dynamically enhances brain responses". primary. Current Biology. 16 (16): 1616–21. doi: 10.1016/j.cub.2006.06.031 . PMID 16920622.
- 1 2 Lucas R (October 2013). "Irradiance Toolbox" (PDF). personalpages.manchester.ac.uk.
- ↑ "International WELL Building Institute". International WELL Building Institute. Retrieved 2018-12-10.
- 1 2 3 4 "Circadian lighting design". WELL Standard. Retrieved 2018-12-10.
- ↑ "Circadian emulation | WELL Standard". standard.wellcertified.com. Retrieved 2018-12-10.
- ↑ Goel N (September 2006). "An arousing, musically enhanced bird song stimulus mediates circadian rhythm phase advances in dim light". primary. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 291 (3): R822–7. doi:10.1152/ajpregu.00550.2005. PMID 16614052.
- ↑ Revell VL, Burgess HJ, Gazda CJ, Smith MR, Fogg LF, Eastman CI (January 2006). "Advancing human circadian rhythms with afternoon melatonin and morning intermittent bright light". primary. The Journal of Clinical Endocrinology and Metabolism. 91 (1): 54–9. doi:10.1210/jc.2005-1009. PMC 3841985 . PMID 16263827.
- ↑ Salazar-Juarez A, Parra-Gamez L, Barbosa Mendez S, Leff P, Anton B (May 2007). "Non-photic entrainment. Another type of entrainment? Part one". Salud Mental. 30 (3): 39–47.
- ↑ Kent MG, Altomonte S, Wilson R, Tregenza PR (2015). "Discomfort glare and time of day". Lighting Research and Technology. 47 (6): 641–657. doi:10.1177/1477153514547291.
- ↑ Kent MG, Altomonte S, Wilson R, Tregenza PR (2016). "Temporal variables and personal factors in glare sensation". Lighting Research and Technology. 48 (6): 689–710. doi:10.1177/1477153515578310.