
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.

Digital microfluidics (DMF) is a platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.

Wetting is the ability of a liquid to maintain contact with a solid surface, resulting from intermolecular interactions when the two are brought together. This happens in presence of a gaseous phase or another liquid phase not miscible with the first one. The degree of wetting (wettability) is determined by a force balance between adhesive and cohesive forces.
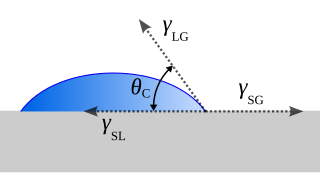
The contact angle is the angle between a liquid surface and a solid surface where they meet. More specifically, it is the angle between the surface tangent on the liquid–vapor interface and the tangent on the solid–liquid interface at their intersection. It quantifies the wettability of a solid surface by a liquid via the Young equation.

The lotus effect refers to self-cleaning properties that are a result of ultrahydrophobicity as exhibited by the leaves of Nelumbo, the lotus flower. Dirt particles are picked up by water droplets due to the micro- and nanoscopic architecture on the surface, which minimizes the droplet's adhesion to that surface. Ultrahydrophobicity and self-cleaning properties are also found in other plants, such as Tropaeolum (nasturtium), Opuntia, Alchemilla, cane, and also on the wings of certain insects.

Cassie's law, or the Cassie equation, describes the effective contact angle θc for a liquid on a chemically heterogeneous surface, i.e. the surface of a composite material consisting of different chemistries, that is non uniform throughout. Contact angles are important as they quantify a surface's wettability, the nature of solid-fluid intermolecular interactions. Cassie's law is reserved for when a liquid completely covers both smooth and rough heterogeneous surfaces.

In chemistry and materials science, ultrahydrophobic surfaces are highly hydrophobic, i.e., extremely difficult to wet. The contact angles of a water droplet on an ultrahydrophobic material exceed 150°. This is also referred to as the lotus effect, after the superhydrophobic leaves of the lotus plant. A droplet striking these kinds of surfaces can fully rebound like an elastic ball. Interactions of bouncing drops can be further reduced using special superhydrophobic surfaces that promote symmetry breaking, pancake bouncing or waterbowl bouncing.

In physics, a "coffee ring" is a pattern left by a puddle of particle-laden liquid after it evaporates. The phenomenon is named for the characteristic ring-like deposit along the perimeter of a spill of coffee. It is also commonly seen after spilling red wine. The mechanism behind the formation of these and similar rings is known as the coffee ring effect or in some instances, the coffee stain effect, or simply ring stain.

Water-in-water (W/W) emulsion is a system that consists of droplets of water-solvated molecules in another continuous aqueous solution; both the droplet and continuous phases contain different molecules that are entirely water-soluble. As such, when two entirely aqueous solutions containing different water-soluble molecules are mixed, water droplets containing predominantly one component are dispersed in water solution containing another component. Recently, such a water-in-water emulsion was demonstrated to exist and be stable from coalescence by the separation of different types of non-amphiphilic, but water-soluble molecular interactions. These molecular interactions include hydrogen bonding, pi stacking, and salt bridging. This w/w emulsion was generated when the different water-solvated molecular functional groups get segregated in an aqueous mixture consisting of polymer and liquid crystal molecules.
A Ramsden emulsion, sometimes named Pickering emulsion, is an emulsion that is stabilized by solid particles which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903.
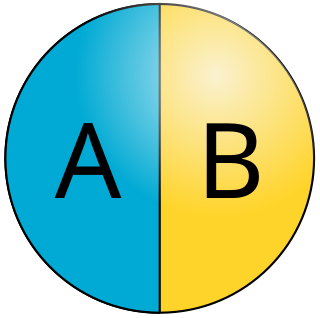
Janus particles are special types of nanoparticles or microparticles whose surfaces have two or more distinct physical properties. This unique surface of Janus particles allows two different types of chemistry to occur on the same particle. The simplest case of a Janus particle is achieved by dividing the particle into two distinct parts, each of them either made of a different material, or bearing different functional groups. For example, a Janus particle may have one-half of its surface composed of hydrophilic groups and the other half hydrophobic groups, the particles might have two surfaces of different color, fluorescence, or magnetic properties. This gives these particles unique properties related to their asymmetric structure and/or functionalization.
A cuticle, or cuticula, is any of a variety of tough but flexible, non-mineral outer coverings of an organism, or parts of an organism, that provide protection. Various types of "cuticle" are non-homologous, differing in their origin, structure, function, and chemical composition.
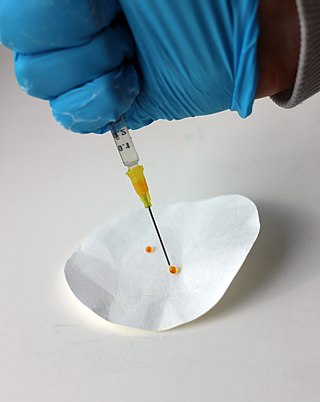
A superhydrophobic coating is a thin surface layer that repels water. It is made from superhydrophobic (ultrahydrophobicity) materials. Droplets hitting this kind of coating can fully rebound. Generally speaking, superhydrophobic coatings are made from composite materials where one component provides the roughness and the other provides low surface energy.
Icephobicity is the ability of a solid surface to repel ice or prevent ice formation due to a certain topographical structure of the surface. The word “icephobic” was used for the first time at least in 1950; however, the progress in micropatterned surfaces resulted in growing interest towards icephobicity since the 2000s.

Edward Bormashenko is a professor of Materials Science and the Head of the Laboratory of Interface Science of the Ariel University in Israel. He was born in 1962 in Kharkiv, Ukraine and lives in Israel since 1997. He studied in the V. N. Karazin Kharkiv National University. His research is in the polymer science and surface science. He accomplished his PhD in Moscow Institute of Plastics in 1990.
Self-cleaning surfaces are a class of materials with the inherent ability to remove any debris or bacteria from their surfaces in a variety of ways. The self-cleaning functionality of these surfaces are commonly inspired by natural phenomena observed in lotus leaves, gecko feet, and water striders to name a few. The majority of self-cleaning surfaces can be placed into three categories:
- superhydrophobic
- superhydrophilic
- photocatalytic.

Droplet cluster is a self-assembled levitating monolayer of microdroplets usually arranged into a hexagonally ordered structure over a locally heated thin layer of water. The droplet cluster is typologically similar to colloidal crystals. The phenomenon was observed for the first time in 2004, and it has been extensively studied after that.
Self-propulsion is the autonomous displacement of nano-, micro- and macroscopic natural and artificial objects, containing their own means of motion. Self-propulsion is driven mainly by interfacial phenomena. Various mechanisms of self-propelling have been introduced and investigated, which exploited phoretic effects, gradient surfaces, breaking the wetting symmetry of a droplet on a surface, the Leidenfrost effect, the self-generated hydrodynamic and chemical fields originating from the geometrical confinements, and soluto- and thermo-capillary Marangoni flows. Self-propelled system demonstrate a potential as micro-fluidics devices and micro-mixers. Self-propelled liquid marbles have been demonstrated.
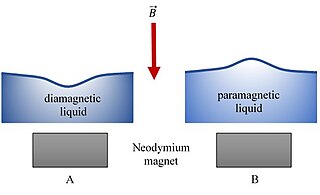
In physics, the Moses effect is a phenomenon of deformation of the surface of a diamagnetic liquid by a magnetic field. The effect was named after the biblical figure Moses, inspired by the mythological crossing of the Red Sea in the Old Testament.

Sylvie Roke is a Dutch chemist and physicist specialized in photonics and aqueous systems. As a full professor she holds Julia Jacobi Chair of Photomedicine at EPFL and is the director of the Laboratory for fundamental BioPhotonics.

















