Related Research Articles

Glass is a non-crystalline, often transparent amorphous solid, that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are "silicate glasses" based on the chemical compound silica, the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term glass, in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material.
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula SiO2, most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries.
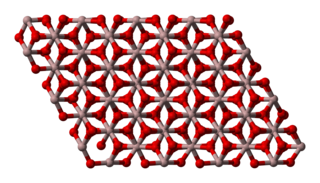
Aluminium oxide is a chemical compound of aluminium and oxygen with the chemical formula Al2O3. It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium(III) oxide. It is commonly called alumina and may also be called aloxide, aloxite, or alundum depending on particular forms or applications. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire. Al2O3 is significant in its use to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point.

Samuel Chao Chung Ting is an American physicist who, with Burton Richter, received the Nobel Prize in 1976 for discovering the subatomic J/ψ particle. More recently he has been the principal investigator in research conducted with the Alpha Magnetic Spectrometer, a device installed on the International Space Station in 2011.

Fused quartz,fused silica or quartz glass is a glass consisting of almost pure silica (silicon dioxide, SiO2) in amorphous (non-crystalline) form. This differs from all other commercial glasses in which other ingredients are added which change the glasses' optical and physical properties, such as lowering the melt temperature. Fused quartz, therefore, has high working and melting temperatures, making it less desirable for most common applications.

Borosilicate glass is a type of glass with silica and boron trioxide as the main glass-forming constituents. Borosilicate glasses are known for having very low coefficients of thermal expansion, making them more resistant to thermal shock than any other common glass. Such glass is subjected to less thermal stress and can withstand temperature differentials without fracturing of about 165 °C (300 °F). It is commonly used for the construction of reagent bottles and flasks as well as lighting, electronics and cookware.
Chalcogenide glass is a glass containing one or more chalcogens. Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties.

Polyamorphism is the ability of a substance to exist in several different amorphous modifications. It is analogous to the polymorphism of crystalline materials. Many amorphous substances can exist with different amorphous characteristics. However, polyamorphism requires two distinct amorphous states with a clear, discontinuous (first-order) phase transition between them. When such a transition occurs between two stable liquid states, a polyamorphic transition may also be referred to as a liquid–liquid phase transition.

Soda–lime glass, also called soda–lime–silica glass, is the most prevalent type of glass, used for windowpanes and glass containers for beverages, food, and some commodity items. Some glass bakeware is made of soda-lime glass, as opposed to the more common borosilicate glass. Soda–lime glass accounts for about 90% of manufactured glass.

Germanium telluride (GeTe) is a chemical compound of germanium and tellurium and is a component of chalcogenide glasses. It shows semimetallic conduction and ferroelectric behaviour.
CLEO was a general purpose particle detector at the Cornell Electron Storage Ring (CESR), and the name of the collaboration of physicists who operated the detector. The name CLEO is not an acronym; it is short for Cleopatra and was chosen to go with CESR. CESR was a particle accelerator designed to collide electrons and positrons at a center-of-mass energy of approximately 10 GeV. The energy of the accelerator was chosen before the first three bottom quark Upsilon resonances were discovered between 9.4 GeV and 10.4 GeV in 1977. The fourth Υ resonance, the Υ(4S), was slightly above the threshold for, and therefore ideal for the study of, B meson production.
Germanium dioxide, also called germanium(IV) oxide, germania, and salt of germanium, is an inorganic compound with the chemical formula GeO2. It is the main commercial source of germanium. It also forms as a passivation layer on pure germanium in contact with atmospheric oxygen.
A frit is a ceramic composition that has been fused, quenched, and granulated. Frits form an important part of the batches used in compounding enamels and ceramic glazes; the purpose of this pre-fusion is to render any soluble and/or toxic components insoluble by causing them to combine with silica and other added oxides. However, not all glass that is fused and quenched in water is frit, as this method of cooling down very hot glass is also widely used in glass manufacture.
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
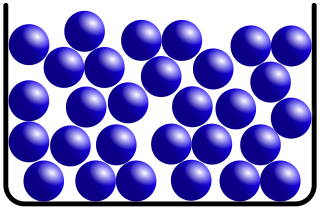
The structure of liquids, glasses and other non-crystalline solids is characterized by the absence of long-range order which defines crystalline materials. Liquids and amorphous solids do, however, possess a rich and varied array of short to medium range order, which originates from chemical bonding and related interactions. Metallic glasses, for example, are typically well described by the dense random packing of hard spheres, whereas covalent systems, such as silicate glasses, have sparsely packed, strongly bound, tetrahedral network structures. These very different structures result in materials with very different physical properties and applications.
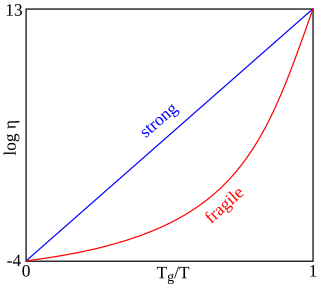
In glass physics, fragility characterizes how rapidly the dynamics of a material slows down as it is cooled toward the glass transition: materials with a higher fragility have a relatively narrow glass transition temperature range, while those with low fragility have a relatively broad glass transition temperature range. Physically, fragility may be related to the presence of dynamical heterogeneity in glasses, as well as to the breakdown of the usual Stokes–Einstein relationship between viscosity and diffusion.

Silicene is a two-dimensional allotrope of silicon, with a hexagonal honeycomb structure similar to that of graphene. Contrary to graphene, silicene is not flat, but has a periodically buckled topology; the coupling between layers in silicene is much stronger than in multilayered graphene; and the oxidized form of silicene, 2D silica, has a very different chemical structure from graphene oxide.

The interface between lanthanum aluminate (LaAlO3) and strontium titanate (SrTiO3) is a notable materials interface because it exhibits properties not found in its constituent materials. Individually, LaAlO3 and SrTiO3 are non-magnetic insulators, yet LaAlO3/SrTiO3 interfaces can exhibit electrical metallic conductivity, superconductivity, ferromagnetism, large negative in-plane magnetoresistance, and giant persistent photoconductivity. The study of how these properties emerge at the LaAlO3/SrTiO3 interface is a growing area of research in condensed matter physics.
Rigidity theory, or topological constraint theory, is a tool for predicting properties of complex networks based on their composition. It was introduced by James Charles Phillips in 1979 and 1981, and refined by Michael Thorpe in 1983. Inspired by the study of the stability of mechanical trusses as pioneered by James Clerk Maxwell, and by the seminal work on glass structure done by William Houlder Zachariasen, this theory reduces complex molecular networks to nodes constrained by rods, thus filtering out microscopic details that ultimately don't affect macroscopic properties. An equivalent theory was developed by P.K. Gupta A.R. Cooper in 1990, where rather than nodes representing atoms, they represented unit polytopes. An example of this would be the SiO tetrahedra in pure glassy silica. This style of analysis has applications in biology and chemistry, such as understanding adaptability in protein-protein interaction networks. Rigidity theory applied to the molecular networks arising from phenotypical expression of certain diseases may provide insights regarding their structure and function.
Hyperuniform materials are mixed-component many-particle systems with unusually low fluctuations in component density at large scales, when compared to the distribution of constituents in common disordered systems, like a mixed ideal gas (air) or typical liquids or amorphous solids: A disordered hyperuniform system is statistically isotropic, like a liquid, but exhibits reduced long-wavelength density fluctuations, similar to crystals.
References
- ↑ "High temperature glass melt property database for process modeling"; Eds.: Thomas P. Seward III and Terese Vascott; The American Ceramic Society, Westerville, Ohio, 2005, ISBN 1-57498-225-7
- ↑ Soda–lime glass for containers is slightly different from soda–lime glass for windows (also called flat glass or float glass). Float glass has a higher magnesium oxide content as compared to container glass, and a lower silica and calcium oxide content. For further details see main article Soda–lime glass.
- ↑ A.J. Leadbettera and A.C. Wrigh (1972). "Diffraction studies of glass structure: II. The structure of vitreous germania". Journal of Non-Crystalline Solids. 7 (1): 37–52. Bibcode:1972JNCS....7...37L. doi:10.1016/0022-3093(72)90016-6.
- ↑ M. Micoulaut; et al. (2006). "Simulated structural and thermal properties of glassy and liquid germania". Physical Review E. 73 (3): 031504. Bibcode:2006PhRvE..73c1504M. doi:10.1103/PhysRevE.73.031504. PMID 16605529.
- ↑ 35 Tg data for GeO2 from SciGlass 6.7
- 1 2 Kotkata; El-Shair, H T; Afifi, M A; Abdel-Aziz, M M; et al. (1994). "Effect of thallium on the optical properties of amorphous GeSe2 and GeSe4 films". J. Phys. D: Appl. Phys. 27 (3): 623–627. Bibcode:1994JPhD...27..623K. doi:10.1088/0022-3727/27/3/031.
- ↑ P. S. Salmon; et al. (2006). "Glass Fragility and Atomic Ordering on the Intermediate and Extended Range". Physical Review Letters. 96 (23): 235502. Bibcode:2006PhRvL..96w5502S. doi:10.1103/PhysRevLett.96.235502. PMID 16803382.
- 1 2 The subscript D indicates that the refractive index n was measured at a wavelength λ of 589.29 nm, F and C indicate 486.13 nm (blue) and 656.27 nm (red) respectively (see article Fraunhofer lines)
- ↑ L. G. Hwa and W.C. Chao (2005). "Velocity of sound and elastic properties of lanthanum gallo-germanate glasses". Materials Chemistry and Physics. 94: 37–41. Bibcode:2005APS..MARU26006H. doi:10.1016/j.matchemphys.2005.04.010.
- ↑ Valid for glass composition, wt%: 80.7 SiO2, 13.1 B2O3, 4.1 Na2O, 2.1 Al2O3; Reference: Baak N. T. E. A. and Rapp C. F., GB Patent No. 1132885 Cl C 03 C 3/04, Abridg. Specif., 1968; Assignee: Owens-Illinois, Inc. (US).
- ↑ International Organization for Standardization, Procedure 719 (1985)