
An alloy is a mixture of chemical elements of which at least one is a metal. Unlike chemical compounds with metallic bases, an alloy will retain all the properties of a metal in the resulting material, such as electrical conductivity, ductility, opacity, and luster, but may have properties that differ from those of the pure metals, such as increased strength or hardness. In some cases, an alloy may reduce the overall cost of the material while preserving important properties. In other cases, the mixture imparts synergistic properties to the constituent metal elements such as corrosion resistance or mechanical strength.
The following outline is provided as an overview of and topical guide to chemistry:

A metal is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile and malleable. These properties are the result of the metallic bond between the atoms or molecules of the metal.

Materials science is an interdisciplinary field of researching and discovering materials. Materials engineering is an engineering field of finding uses for materials in other fields and industries.

Metallurgy is a domain of materials science and engineering that studies the physical and chemical behavior of metallic elements, their inter-metallic compounds, and their mixtures, which are known as alloys.
In materials science, a metal matrix composite (MMC) is a composite material with fibers or particles dispersed in a metallic matrix, such as copper, aluminum, or steel. The secondary phase is typically a ceramic or another metal. They are typically classified according to the type of reinforcement: short discontinuous fibers (whiskers), continuous fibers, or particulates. There is some overlap between MMCs and cermets, with the latter typically consisting of less than 20% metal by volume. When at least three materials are present, it is called a hybrid composite. MMCs can have much higher strength-to-weight ratios, stiffness, and ductility than traditional materials, so they are often used in demanding applications. MMCs typically have lower thermal and electrical conductivity and poor resistance to radiation, limiting their use in the very harshest environments.
Thermal analysis is a branch of materials science where the properties of materials are studied as they change with temperature. Several methods are commonly used – these are distinguished from one another by the property which is measured:

Foams are materials formed by trapping pockets of gas in a liquid or solid.

Soft matter or soft condensed matter is a subfield of condensed matter comprising a variety of physical systems that are deformed or structurally altered by thermal or mechanical stress of the magnitude of thermal fluctuations. These materials share an important common feature in that predominant physical behaviors occur at an energy scale comparable with room temperature thermal energy, and that entropy is considered the dominant factor. At these temperatures, quantum aspects are generally unimportant. Soft materials include liquids, colloids, polymers, foams, gels, granular materials, liquid crystals, flesh, and a number of biomaterials. When soft materials interact favorably with surfaces, they become squashed without an external compressive force. Pierre-Gilles de Gennes, who has been called the "founding father of soft matter," received the Nobel Prize in Physics in 1991 for discovering that methods developed for studying order phenomena in simple systems can be generalized to the more complex cases found in soft matter, in particular, to the behaviors of liquid crystals and polymers.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.
Titanium alloys are alloys that contain a mixture of titanium and other chemical elements. Such alloys have very high tensile strength and toughness. They are light in weight, have extraordinary corrosion resistance and the ability to withstand extreme temperatures. However, the high cost of both raw materials and processing limit their use to military applications, aircraft, spacecraft, bicycles, medical devices, jewelry, highly stressed components such as connecting rods on expensive sports cars and some premium sports equipment and consumer electronics.

In materials science, a metal foam is a material or structure consisting of a solid metal with gas-filled pores comprising a large portion of the volume. The pores can be sealed or interconnected. The defining characteristic of metal foams is a high porosity: typically only 5–25% of the volume is the base metal. The strength of the material is due to the square–cube law.

Ceramic engineering is the science and technology of creating objects from inorganic, non-metallic materials. This is done either by the action of heat, or at lower temperatures using precipitation reactions from high-purity chemical solutions. The term includes the purification of raw materials, the study and production of the chemical compounds concerned, their formation into components and the study of their structure, composition and properties.

An aluminium alloy (UK/IUPAC) or aluminum alloy is an alloy in which aluminium (Al) is the predominant metal. The typical alloying elements are copper, magnesium, manganese, silicon, tin, nickel and zinc. There are two principal classifications, namely casting alloys and wrought alloys, both of which are further subdivided into the categories heat-treatable and non-heat-treatable. About 85% of aluminium is used for wrought products, for example rolled plate, foils and extrusions. Cast aluminium alloys yield cost-effective products due to the low melting point, although they generally have lower tensile strengths than wrought alloys. The most important cast aluminium alloy system is Al–Si, where the high levels of silicon (4–13%) contribute to give good casting characteristics. Aluminium alloys are widely used in engineering structures and components where light weight or corrosion resistance is required.
Ceramic foam is a tough foam made from ceramics. Manufacturing techniques include impregnating open-cell polymer foams internally with ceramic slurry and then firing in a kiln, leaving only ceramic material. The foams may consist of several ceramic materials such as aluminium oxide, a common high-temperature ceramic, and gets insulating properties from the many tiny air-filled voids within the material.
Radiation damage is the effect of ionizing radiation on physical objects including non-living structural materials. It can be either detrimental or beneficial for materials.

Solid is one of the four fundamental states of matter along with liquid, gas, and plasma. The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice, or irregularly. Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed.
Ultralight materials are solids with a density of less than 10 mg/cm3, including silica aerogels, carbon nanotube aerogels, aerographite, metallic foams, polymeric foams, and metallic microlattices. The density of air is about 1.275 mg/cm3, which means that the air in the pores contributes significantly to the density of these materials in atmospheric conditions. They can be classified by production method as aerogels, stochastic foams, and structured cellular materials.
Titanium foams exhibit high specific strength, high energy absorption, excellent corrosion resistance and biocompatibility. These materials are ideally suited for applications within the aerospace industry. An inherent resistance to corrosion allows the foam to be a desirable candidate for various filtering applications. Further, titanium's physiological inertness makes its porous form a promising candidate for biomedical implantation devices. The largest advantage in fabricating titanium foams is that the mechanical and functional properties can be adjusted through manufacturing manipulations that vary porosity and cell morphology. The high appeal of titanium foams is directly correlated to a multi-industry demand for advancement in this technology.
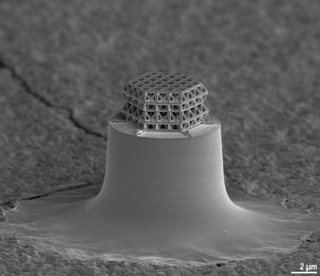
A nanolattice is a synthetic porous material consisting of nanometer-size members patterned into an ordered lattice structure, like a space frame. The nanolattice is a newly emerged material class that has been rapidly developed over the last decade. Nanolattices redefine the limits of the material property space. Despite being composed of 50-99% of air, nanolattices are very mechanically robust because they take advantage of size-dependent properties that we generally see in nanoparticles, nanowires, and thin films. The most typical mechanical properties of nanolattices include ultrahigh strength, damage tolerance, and high stiffness. Thus, nanolattices have a wide range of applications.













