Related Research Articles
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of a molecule. Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy.

Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic field. This re-orientation occurs with absorption of electromagnetic radiation in the radio frequency region from roughly 4 to 900 MHz, which depends on the isotopic nature of the nucleus and increased proportionally to the strength of the external magnetic field. Notably, the resonance frequency of each NMR-active nucleus depends on its chemical environment. As a result, NMR spectra provide information about individual functional groups present in the sample, as well as about connections between nearby nuclei in the same molecule. As the NMR spectra are unique or highly characteristic to individual compounds and functional groups, NMR spectroscopy is one of the most important methods to identify molecular structures, particularly of organic compounds.
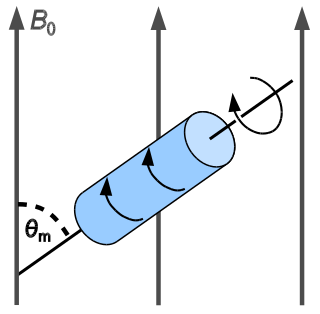
In solid-state NMR spectroscopy, magic-angle spinning (MAS) is a technique routinely used to produce better resolution NMR spectra. MAS NMR consists in spinning the sample at the magic angle θm with respect to the direction of the magnetic field.

Solid-state NMR (ssNMR) spectroscopy is a technique for characterizing atomic level structure in solid materials e.g. powders, single crystals and amorphous samples and tissues using nuclear magnetic resonance (NMR) spectroscopy. The anisotropic part of many spin interactions are present in solid-state NMR, unlike in solution-state NMR where rapid tumbling motion averages out many of the spin interactions. As a result, solid-state NMR spectra are characterised by larger linewidths than in solution state NMR, which can be utilized to give quantitative information on the molecular structure, conformation and dynamics of the material. Solid-state NMR is often combined with magic angle spinning to remove anisotropic interactions and improve the resolution as well as the sensitivity of the technique.

Angewandte Chemie is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society. Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the Journal Citation Reports, the journal had a 2021 impact factor of 16.823.
Jonathan Paul Clayden is a Professor of organic chemistry at the University of Bristol.

The fluoronium ion is an inorganic cation with the chemical formula H
2F+
. It is one of the cations found in fluoroantimonic acid. The structure of the salt with the Sb
2F−
11 anion, has been determined. The fluoronium ion is isoelectronic with the water molecule and the azanide ion.
Schlosser's base describes various superbasic mixtures of an alkyllithium compound and a potassium alkoxide. The reagent is named after Manfred Schlosser, although he uses the term LICKOR superbase. The superbasic nature of the reagent is a consequence of the in situ formation of the corresponding organopotassium compound, as well as changes to the aggregation state of the alkyllithium species.

Fluorine-19 nuclear magnetic resonance spectroscopy is an analytical technique used to detect and identify fluorine-containing compounds. 19F is an important nucleus for NMR spectroscopy because of its receptivity and large chemical shift dispersion, which is greater than that for proton nuclear magnetic resonance spectroscopy.
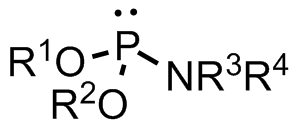
A phosphoramidite (RO)2PNR2 is a monoamide of a phosphite diester. The key feature of phosphoramidites is their markedly high reactivity towards nucleophiles catalyzed by weak acids e.c., triethylammonium chloride or 1H-tetrazole. In these reactions, the incoming nucleophile replaces the NR2 moiety.
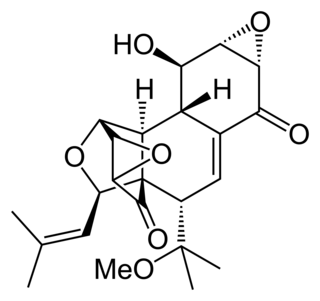
Hexacyclinol is a natural metabolite of a fungus, Panus rudis. Significant controversy surrounded its proposed structure until its total synthesis by John Porco, Jr. in 2006.
Nuclear magnetic resonance crystallography is a method which utilizes primarily NMR spectroscopy to determine the structure of solid materials on the atomic scale. Thus, solid-state NMR spectroscopy would be used primarily, possibly supplemented by quantum chemistry calculations, powder diffraction etc. If suitable crystals can be grown, any crystallographic method would generally be preferred to determine the crystal structure comprising in case of organic compounds the molecular structures and molecular packing. The main interest in NMR crystallography is in microcrystalline materials which are amenable to this method but not to X-ray, neutron and electron diffraction. This is largely because interactions of comparably short range are measured in NMR crystallography.

Paramagnetic nuclear magnetic resonance spectroscopy refers to nuclear magnetic resonance (NMR) spectroscopy of paramagnetic compounds. Although most NMR measurements are conducted on diamagnetic compounds, paramagnetic samples are also amenable to analysis and give rise to special effects indicated by a wide chemical shift range and broadened signals. Paramagnetism diminishes the resolution of an NMR spectrum to the extent that coupling is rarely resolved. Nonetheless spectra of paramagnetic compounds provide insight into the bonding and structure of the sample. For example, the broadening of signals is compensated in part by the wide chemical shift range (often 200 ppm in 1H NMR). Since paramagnetism leads to shorter relaxation times (T1), the rate of spectral acquisition can be high.

Gareth Alun Morris FRS is a Professor of Physical Chemistry, in the School of Chemistry at the University of Manchester.
Paul Knochel is a French chemist and a member of the French Academy of Sciences.
David John Procter is a British chemist and a Professor in the Department of Chemistry at The University of Manchester. His research is based on organic chemistry and catalysis, specifically on radical cascades, sulfonium cross-coupling and copper catalysis.
Aluminium(I) nucleophiles are a group of inorganic and organometallic nucleophilic compounds containing at least one aluminium metal center in the +1 oxidation state with a lone pair of electrons strongly localized on the aluminium(I) center.
The borosulfates are heteropoly anion compounds which have sulfate groups attached to boron atoms. Other possible terms are sulfatoborates or boron-sulfur oxides. The ratio of sulfate to borate reflects the degree of condensation. With [B(SO4)4]5- there is no condensation, each ion stands alone. In [B(SO4)3]3- the anions are linked into a chain, a chain of loops, or as [B2(SO4)6]6− in a cycle. Finally in [B(SO4)2]− the sulfate and borate tetrahedra are all linked into a two or three-dimensional network. These arrangements of oxygen around boron and sulfur can have forms resembling silicates. The first borosulfate to be discovered was K5[B(SO4)4] in 2012. Over 75 unique compounds are known.
The methods for sequence analysis of synthetic polymers differ from the sequence analysis of biopolymers. Synthetic polymers are produced by chain-growth or step-growth polymerization and show thereby polydispersity, whereas biopolymers are synthesized by complex template-based mechanisms and are sequence-defined and monodisperse. Synthetic polymers are a mixture of macromolecules of different length and sequence and are analysed via statistical measures.

Aluminylenes are a sub-class of aluminium(I) compounds that feature singly-coordinated aluminium atoms with a lone pair of electrons. As aluminylenes exhibit two unoccupied orbitals, they are not strictly aluminium analogues of carbenes until stabilized by a Lewis base to form aluminium(I) nucleophiles. The lone pair and two empty orbitals on the aluminium allow for ambiphilic bonding where the aluminylene can act as both an electrophile and a nucleophile. Aluminylenes have also been reported under the names alumylenes and alanediyl.
References
- 1 2 3 4 University of Manchester. "Prof. Mathias Nilsson" . Retrieved 16 June 2020.
- 1 2 Institute of Physics. "The BRSG-NMRDG Annual Prize" . Retrieved 16 June 2020.
- 1 2 "Prof. M. Nilsson: Publications" . Retrieved 16 June 2020.
- 1 2 Mathias, Nisson (1999). The dietary fibre complex of rye grain, with emphasis on arabinoxylan (PhD thesis).(subscription required)
- 1 2 3 University of Manchester NMR Group. "Prof. Mathias Nilsson (NMR Group at University of ManchesteR)" . Retrieved 16 June 2020.
- ↑ University of Manchester NMR Group. "People: NMR Group" . Retrieved 16 June 2020.
- ↑ University of Manchester NMR Group. "Workshop on pure shift NMR" . Retrieved 16 June 2020.
- ↑ ANZMAG. "Inivted Speakers (2019)" . Retrieved 16 June 2020.
- ↑ Nilsson, Mathias; Aguilar, Juan A.; Faulkner, Stephen; Morris, Gareth A. (2010). "Pure Shift 1H NMR: A Resolution of the Resolution Problem?". Angewandte Chemie International Edition. 49 (23): 3901–3903. doi:10.1002/anie.201001107. PMID 20401889 . Retrieved 16 June 2020.
- ↑ Castañar, Laura (2017). "Pure shift 1H NMR: what is next?". Special Issue: Perspectives on the Future of NMR. 55 (1): 47–53. doi:10.1002/mrc.4545. PMID 27761957. S2CID 3708076 . Retrieved 18 June 2020.
- ↑ Nilsson, Mathias (2009). "The DOSY Toolbox: A new tool for processing PFG NMR diffusion data". Journal of Magnetic Resonance. 200 (2): 296–302. Bibcode:2009JMagR.200..296N. doi:10.1016/j.jmr.2009.07.022. PMID 19666235 . Retrieved 16 June 2020.