Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
The following outline is provided as an overview of and topical guide to chemistry:
Physical science is a branch of natural science that studies non-living systems, in contrast to life science. It in turn has many branches, each referred to as a "physical science", together is called the "physical sciences".
Quantum chemistry, also called molecular quantum mechanics, is a branch of physical chemistry focused on the application of quantum mechanics to chemical systems, particularly towards the quantum-mechanical calculation of electronic contributions to physical and chemical properties of molecules, materials, and solutions at the atomic level. These calculations include systematically applied approximations intended to make calculations computationally feasible while still capturing as much information about important contributions to the computed wave functions as well as to observable properties such as structures, spectra, and thermodynamic properties. Quantum chemistry is also concerned with the computation of quantum effects on molecular dynamics and chemical kinetics.
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applications include many problems in the fields of physics, biology, chemistry, neuroscience, computer science, information theory and sociology. Its main purpose is to clarify the properties of matter in aggregate, in terms of physical laws governing atomic motion.

Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics which convey a quantitative description using measurable macroscopic physical quantities, but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering and mechanical engineering, but also in other complex fields such as meteorology.

Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface of potential energy, molecular orbitals, orbital interactions, and molecule activation.

A thermodynamic system is a body of matter and/or radiation separate from its surroundings that can be studied using the laws of thermodynamics.
In chemistry, reactivity is the impulse for which a chemical substance undergoes a chemical reaction, either by itself or with other materials, with an overall release of energy.

In chemistry, an activated complex represents a collection of intermediate structures in a chemical reaction when bonds are breaking and forming. The activated complex is an arrangement of atoms in an arbitrary region near the saddle point of a potential energy surface. The region represents not one defined state, but a range of unstable configurations that a collection of atoms pass through between the reactants and products of a reaction. Activated complexes have partial reactant and product character, which can significantly impact their behaviour in chemical reactions.
Chemical physics is a subdiscipline of chemistry and physics that investigates physicochemical phenomena using techniques from atomic and molecular physics and condensed matter physics; it is the branch of physics that studies chemical processes from the point of view of physics. While at the interface of physics and chemistry, chemical physics is distinct from physical chemistry in that it focuses more on the characteristic elements and theories of physics. Meanwhile, physical chemistry studies the physical nature of chemistry. Nonetheless, the distinction between the two fields is vague, and scientists often practice in both fields during the course of their research.
The scientific school of Quantum electrochemistry began to form in the 1960s under Revaz Dogonadze. Generally speaking, the field comprises the notions arising in electrodynamics, quantum mechanics, and electrochemistry; and so is studied by a very large array of different professional researchers. The fields they reside in include, chemical, electrical and mechanical engineering, chemistry and physics.

A potential energy surface (PES) or energy landscape describes the energy of a system, especially a collection of atoms, in terms of certain parameters, normally the positions of the atoms. The surface might define the energy as a function of one or more coordinates; if there is only one coordinate, the surface is called a potential energy curve or energy profile. An example is the Morse/Long-range potential.
In thermodynamics, the interpretation of entropy as a measure of energy dispersal has been exercised against the background of the traditional view, introduced by Ludwig Boltzmann, of entropy as a quantitative measure of disorder. The energy dispersal approach avoids the ambiguous term 'disorder'. An early advocate of the energy dispersal conception was Edward A. Guggenheim in 1949, using the word 'spread'.
Physical organic chemistry, a term coined by Louis Hammett in 1940, refers to a discipline of organic chemistry that focuses on the relationship between chemical structures and reactivity, in particular, applying experimental tools of physical chemistry to the study of organic molecules. Specific focal points of study include the rates of organic reactions, the relative chemical stabilities of the starting materials, reactive intermediates, transition states, and products of chemical reactions, and non-covalent aspects of solvation and molecular interactions that influence chemical reactivity. Such studies provide theoretical and practical frameworks to understand how changes in structure in solution or solid-state contexts impact reaction mechanism and rate for each organic reaction of interest.
Reaction dynamics is a field within physical chemistry, studying why chemical reactions occur, how to predict their behavior, and how to control them. It is closely related to chemical kinetics, but is concerned with individual chemical events on atomic length scales and over very brief time periods. It considers state-to-state kinetics between reactant and product molecules in specific quantum states, and how energy is distributed between translational, vibrational, rotational, and electronic modes.
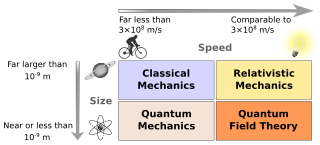
Physics is a scientific discipline that seeks to construct and experimentally test theories of the physical universe. These theories vary in their scope and can be organized into several distinct branches, which are outlined in this article.
Food physical chemistry is considered to be a branch of Food chemistry concerned with the study of both physical and chemical interactions in foods in terms of physical and chemical principles applied to food systems, as well as the applications of physical/chemical techniques and instrumentation for the study of foods. This field encompasses the "physiochemical principles of the reactions and conversions that occur during the manufacture, handling, and storage of foods."
The following outline is provided as an overview of and topical guide to natural science:
In physical chemistry, there are numerous quantities associated with chemical compounds and reactions; notably in terms of amounts of substance, activity or concentration of a substance, and the rate of reaction. This article uses SI units.








