The actinide or actinoid series encompasses the 14 metallic chemical elements with atomic numbers from 89 to 103, actinium through Lawrencium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Polonium is a chemical element; it has symbol Po and atomic number 84. A rare and highly radioactive metal with no stable isotopes, polonium is a chalcogen and chemically similar to selenium and tellurium, though its metallic character resembles that of its horizontal neighbors in the periodic table: thallium, lead, and bismuth. Due to the short half-life of all its isotopes, its natural occurrence is limited to tiny traces of the fleeting polonium-210 in uranium ores, as it is the penultimate daughter of natural uranium-238. Though longer-lived isotopes exist, such as the 125.2 years half-life of polonium-209, they are much more difficult to produce. Today, polonium is usually produced in milligram quantities by the neutron irradiation of bismuth. Due to its intense radioactivity, which results in the radiolysis of chemical bonds and radioactive self-heating, its chemistry has mostly been investigated on the trace scale only.

Uranium is a chemical element; it has symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium radioactively decays by emitting an alpha particle. The half-life of this decay varies between 159,200 and 4.5 billion years for different isotopes, making them useful for dating the age of the Earth. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite.
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuclide the decay rate, and thus the half-life (t1/2) for that collection, can be calculated from their measured decay constants. The range of the half-lives of radioactive atoms has no known limits and spans a time range of over 55 orders of magnitude.

Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment.
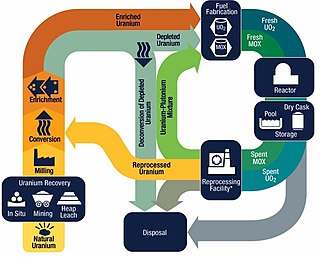
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle ; if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.

Radioactive decay is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered radioactive. Three of the most common types of decay are alpha, beta, and gamma decay. The weak force is the mechanism that is responsible for beta decay, while the other two are governed by the electromagnetism and nuclear force.

In nuclear science, the decay chain refers to a series of radioactive decays of different radioactive decay products as a sequential series of transformations. It is also known as a "radioactive cascade". The typical radioisotope does not decay directly to a stable state, but rather it decays to another radioisotope. Thus there is usually a series of decays until the atom has become a stable isotope, meaning that the nucleus of the atom has reached a stable state.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
Polonium-210 (210Po, Po-210, historically radium F) is an isotope of polonium. It undergoes alpha decay to stable 206Pb with a half-life of 138.376 days (about 4+1⁄2 months), the longest half-life of all naturally occurring polonium isotopes (210–218Po). First identified in 1898, and also marking the discovery of the element polonium, 210Po is generated in the decay chain of uranium-238 and radium-226. 210Po is a prominent contaminant in the environment, mostly affecting seafood and tobacco. Its extreme toxicity is attributed to intense radioactivity, mostly due to alpha particles, which easily cause radiation damage, including cancer in surrounding tissue. The specific activity of 210
Po is 166 TBq/g, i.e., 1.66 × 1014 Bq/g. At the same time, 210Po is not readily detected by common radiation detectors, because its gamma rays have a very low energy. Therefore, 210
Po can be considered as a quasi-pure alpha emitter.

Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons. Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.
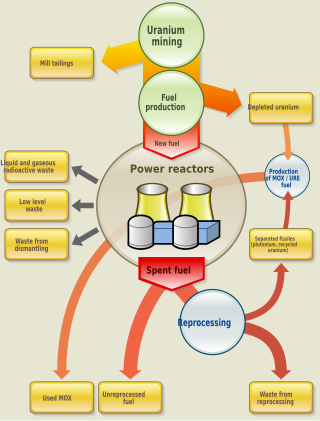
Nuclear fuel is material used in nuclear power stations to produce heat to power turbines. Heat is created when nuclear fuel undergoes nuclear fission.
Neptunium (93Np) is usually considered an artificial element, although trace quantities are found in nature, so a standard atomic weight cannot be given. Like all trace or artificial elements, it has no stable isotopes. The first isotope to be synthesized and identified was 239Np in 1940, produced by bombarding 238
U
with neutrons to produce 239
U
, which then underwent beta decay to 239
Np
.
Plutonium (94Pu) is an artificial element, except for trace quantities resulting from neutron capture by uranium, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no stable isotopes. It was synthesized long before being found in nature, the first isotope synthesized being 238Pu in 1940. Twenty plutonium radioisotopes have been characterized. The most stable are plutonium-244 with a half-life of 80.8 million years, plutonium-242 with a half-life of 373,300 years, and plutonium-239 with a half-life of 24,110 years. All of the remaining radioactive isotopes have half-lives that are less than 7,000 years. This element also has eight meta states; all have half-lives of less than one second.
Americium (95Am) is an artificial element, and thus a standard atomic weight cannot be given. Like all artificial elements, it has no known stable isotopes. The first isotope to be synthesized was 241Am in 1944. The artificial element decays by ejecting alpha particles. Americium has an atomic number of 95. Despite 243
Am being an order of magnitude longer lived than 241
Am, the former is harder to obtain than the latter as more of it is present in spent nuclear fuel.

Environmental radioactivity is produced by radioactive materials in the human environment. While some radioisotopes, such as strontium-90 (90Sr) and technetium-99 (99Tc), are only found on Earth as a result of human activity, and some, like potassium-40 (40K), are only present due to natural processes, a few isotopes, e.g. tritium (3H), result from both natural processes and human activities. The concentration and location of some natural isotopes, particularly uranium-238 (238U), can be affected by human activity.

Since the mid-20th century, plutonium in the environment has been primarily produced by human activity. The first plants to produce plutonium for use in cold war atomic bombs were at the Hanford nuclear site, in Washington, and Mayak nuclear plant, in Chelyabinsk Oblast, Russia. Over a period of four decades, "both released more than 200 million curies of radioactive isotopes into the surrounding environment – twice the amount expelled in the Chernobyl disaster in each instance".

Isotopes are distinct nuclear species of the same chemical element. They have the same atomic number and position in the periodic table, but differ in nucleon numbers due to different numbers of neutrons in their nuclei. While all isotopes of a given element have almost the same chemical properties, they have different atomic masses and physical properties.
The discovery of the neutron and its properties was central to the extraordinary developments in atomic physics in the first half of the 20th century. Early in the century, Ernest Rutherford developed a crude model of the atom, based on the gold foil experiment of Hans Geiger and Ernest Marsden. In this model, atoms had their mass and positive electric charge concentrated in a very small nucleus. By 1920, isotopes of chemical elements had been discovered, the atomic masses had been determined to be (approximately) integer multiples of the mass of the hydrogen atom, and the atomic number had been identified as the charge on the nucleus. Throughout the 1920s, the nucleus was viewed as composed of combinations of protons and electrons, the two elementary particles known at the time, but that model presented several experimental and theoretical contradictions.