Actinium is a chemical element; it has symbol Ac and atomic number 89. It was first isolated by Friedrich Oskar Giesel in 1902, who gave it the name emanium; the element got its name by being wrongly identified with a substance André-Louis Debierne found in 1899 and called actinium. Actinium gave the name to the actinide series, a set of 15 elements between actinium and lawrencium in the periodic table. Together with polonium, radium, and radon, actinium was one of the first non-primordial radioactive elements to be isolated.

Americium is a synthetic chemical element; it has symbol Am and atomic number 95. It is radioactive and a transuranic member of the actinide series in the periodic table, located under the lanthanide element europium and was thus named after the Americas by analogy.
The actinide or actinoid series encompasses at least the 14 metallic chemical elements in the 5f series, with atomic numbers from 89 to 102, actinium through nobelium. The actinide series derives its name from the first element in the series, actinium. The informal chemical symbol An is used in general discussions of actinide chemistry to refer to any actinide.

Curium is a synthetic chemical element; it has symbol Cm and atomic number 96. This transuranic actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first intentionally made by the team of Glenn T. Seaborg, Ralph A. James, and Albert Ghiorso in 1944, using the cyclotron at Berkeley. They bombarded the newly discovered element plutonium with alpha particles. This was then sent to the Metallurgical Laboratory at University of Chicago where a tiny sample of curium was eventually separated and identified. The discovery was kept secret until after the end of World War II. The news was released to the public in November 1947. Most curium is produced by bombarding uranium or plutonium with neutrons in nuclear reactors – one tonne of spent nuclear fuel contains ~20 grams of curium.

Thorium is a chemical element. It has the symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive gray when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided.

Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the cerium-dominant member of the group. It occurs usually in small isolated crystals. It has a hardness of 5.0 to 5.5 on the Mohs scale of mineral hardness and is relatively dense, about 4.6 to 5.7 g/cm3. There are five different most common species of monazite, depending on the relative amounts of the rare earth elements in the mineral:

Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium, also known as the spent fuel material, can in principle also be re-used as fuel, but that is only economical when uranium supply is low and prices are high. Nuclear reprocessing may extend beyond fuel and include the reprocessing of other nuclear reactor material, such as Zircaloy cladding.
A period 7 element is one of the chemical elements in the seventh row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases: a new row is begun when chemical behavior begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The seventh period contains 32 elements, tied for the most with period 6, beginning with francium and ending with oganesson, the heaviest element currently discovered. As a rule, period 7 elements fill their 7s shells first, then their 5f, 6d, and 7p shells in that order, but there are exceptions, such as uranium.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
Tributyl phosphate, known commonly as TBP, is an organophosphorus compound with the chemical formula (CH3CH2CH2CH2O)3PO. This colourless, odorless liquid finds some applications as an extractant and a plasticizer. It is an ester of phosphoric acid with n-butanol.
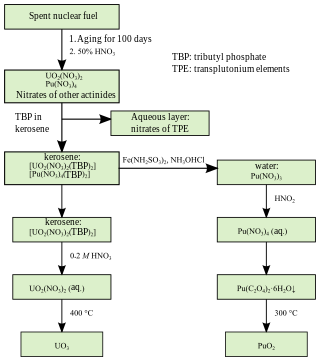
PUREX is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the de facto standard aqueous nuclear reprocessing method for the recovery of uranium and plutonium from used nuclear fuel. It is based on liquid–liquid extraction ion-exchange.

Liquid–liquid extraction, also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration. The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. Liquid-liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed after a chemical reaction as part of the work-up, often including an acidic work-up.
The uranyl ion is an oxycation of uranium in the oxidation state +6, with the chemical formula UO2+
2. It has a linear structure with short U–O bonds, indicative of the presence of multiple bonds between uranium and oxygen. Four or more ligands may be bound to the uranyl ion in an equatorial plane around the uranium atom. The uranyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Complexes of the uranyl ion are important in the extraction of uranium from its ores and in nuclear fuel reprocessing.

Environmental radioactivity is not limited to actinides; non-actinides such as radon and radium are of note. While all actinides are radioactive, there are a lot of actinides or actinide-relating minerals in the Earth's crust such as uranium and thorium. These minerals are helpful in many ways, such as carbon-dating, most detectors, X-rays, and more.
In nuclear chemistry, the actinide concept proposed that the actinides form a second inner transition series homologous to the lanthanides. Its origins stem from observation of lanthanide-like properties in transuranic elements in contrast to the distinct complex chemistry of previously known actinides. Glenn Theodore Seaborg, one of the researchers who synthesized transuranic elements, proposed the actinide concept in 1944 as an explanation for observed deviations and a hypothesis to guide future experiments. It was accepted shortly thereafter, resulting in the placement of a new actinide series comprising elements 89 (actinium) to 103 (lawrencium) below the lanthanides in Dmitri Mendeleev's periodic table of the elements.

Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond.
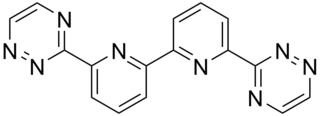
The bis-triazinyl bipyridines (BTBPs) are a class of chemical compounds which are tetradentate ligands similar in shape to quaterpyridine. The BTBPs are made by the reaction of hydrazine and a 1,2-diketone with 6,6'-dicyano-2,2'-bipyridine. The dicyanobipy can be made by reacting 2,2'-bipy with hydrogen peroxide in acetic acid, to form 2,2'-bipyridine-N,N-dioxide. The 2,2'-bipyridine-N,N-dioxide is then converted into the dicyano compound by treatment with potassium cyanide and benzoyl chloride in a mixture of water and THF.
The plutonyl ion is an oxycation of plutonium in the oxidation state +6, with the chemical formula PuO2+
2. It is isostructural with the uranyl ion, compared to which it has a slightly shorter M–O bond. It is easily reduced to plutonium(III). The plutonyl ion forms many complexes, particularly with ligands that have oxygen donor atoms. Plutonyl salts are important in nuclear fuel reprocessing.

Many compounds of thorium are known: this is because thorium and uranium are the most stable and accessible actinides and are the only actinides that can be studied safely and legally in bulk in a normal laboratory. As such, they have the best-known chemistry of the actinides, along with that of plutonium, as the self-heating and radiation from them is not enough to cause radiolysis of chemical bonds as it is for the other actinides. While the later actinides from americium onwards are predominantly trivalent and behave more similarly to the corresponding lanthanides, as one would expect from periodic trends, the early actinides up to plutonium have relativistically destabilised and hence delocalised 5f and 6d electrons that participate in chemistry in a similar way to the early transition metals of group 3 through 8: thus, all their valence electrons can participate in chemical reactions, although this is not common for neptunium and plutonium.
The advanced reprocessing of spent nuclear fuel is a potential key to achieve a sustainable nuclear fuel cycle and to tackle the heavy burden of nuclear waste management. In particular, the development of such advanced reprocessing systems may save natural resources, reduce waste inventory and enhance the public acceptance of nuclear energy. This strategy relies on the recycling of major actinides and the transmutation of minor actinides in appropriate reactors. In order to fulfill this objective, selective extracting agents need to be designed and developed by investigating their complexation mechanism.














