In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.
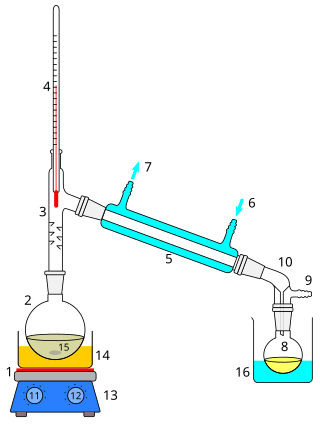
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.
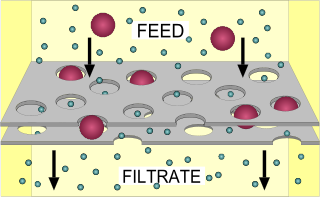
Filtration is a physical separation process that separates solid matter and fluid from a mixture using a filter medium that has a complex structure through which only the fluid can pass. Solid particles that cannot pass through the filter medium are described as oversize and the fluid that passes through is called the filtrate. Oversize particles may form a filter cake on top of the filter and may also block the filter lattice, preventing the fluid phase from crossing the filter, known as blinding. The size of the largest particles that can successfully pass through a filter is called the effective pore size of that filter. The separation of solid and fluid is imperfect; solids will be contaminated with some fluid and filtrate will contain fine particles. Filtration occurs both in nature and in engineered systems; there are biological, geological, and industrial forms.

In physics, a vapor or vapour is a substance in the gas phase at a temperature lower than its critical temperature, which means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from an aerosol. An aerosol is a suspension of tiny particles of liquid, solid, or both within a gas.
Refining is the process of purification of a (1) substance or a (2) form. The term is usually used of a natural resource that is almost in a usable form, but which is more useful in its pure form. For instance, most types of natural petroleum will burn straight from the ground, but it will burn poorly and quickly clog an engine with residues and by-products. The term is broad, and may include more drastic transformations, such as the reduction of ore to metal.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

Fractionation is a separation process in which a certain quantity of a mixture is divided during a phase transition, into a number of smaller quantities (fractions) in which the composition varies according to a gradient. Fractions are collected based on differences in a specific property of the individual components. A common trait in fractionations is the need to find an optimum between the amount of fractions collected and the desired purity in each fraction. Fractionation makes it possible to isolate more than two components in a mixture in a single run. This property sets it apart from other separation techniques.

In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomerization, and other reactions. For example, in milk processing, the following unit operations are involved: homogenization, pasteurization, and packaging. These unit operations are connected to create the overall process. A process may require many unit operations to obtain the desired product from the starting materials, or feedstocks.
Petroleum geochemistry is a branch of geochemistry which deals specifically with petroleum and its origin, generation, and accumulation, as well as its extraction, refinement, and use. Petroleum, also known as crude oil, is a solid, liquid, and/or gaesous mix of hydrocarbons. These hydrocarbons are from the burial and metamorphosis of organic matter from millions of years ago; the organic matter is from marine animals, plants, and algae. Petroleum is extracted from the Earth, refined, and used as an energy source.

In chemistry, volatility is a material quality which describes how readily a substance vaporizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate such as dry ice or iodine can vaporize at a similar rate as some liquids under standard conditions.

Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
A zeotropicmixture, or non-azeotropic mixture, is a mixture with liquid components that have different boiling points. For example, nitrogen, methane, ethane, propane, and isobutane constitute a zeotropic mixture. Individual substances within the mixture do not evaporate or condense at the same temperature as one substance. In other words, the mixture has a temperature glide, as the phase change occurs in a temperature range of about four to seven degrees Celsius, rather than at a constant temperature. On temperature-composition graphs, this temperature glide can be seen as the temperature difference between the bubble point and dew point. For zeotropic mixtures, the temperatures on the bubble (boiling) curve are between the individual component's boiling temperatures. When a zeotropic mixture is boiled or condensed, the composition of the liquid and the vapor changes according to the mixtures's temperature-composition diagram.
Acid–base extraction is a subclass of liquid–liquid extractions and involves the separation of chemical species from other acidic or basic compounds. It is typically performed during the work-up step following a chemical synthesis to purify crude compounds and results in the product being largely free of acidic or basic impurities. A separatory funnel is commonly used to perform an acid-base extraction.

In chemistry, a condenser is laboratory apparatus used to condense vapors – that is, turn them into liquids – by cooling them down.
The history of chromatography spans from the mid-19th century to the 21st. Chromatography, literally "color writing", was used—and named— in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll and carotenoids. New forms of chromatography developed in the 1930s and 1940s made the technique useful for a wide range of separation processes and chemical analysis tasks, especially in biochemistry.

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by inertia of the molecules composing the stationary phase accelerating toward the center of a centrifuge due to centripetal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.
Industrial separation processes are technical procedures which are used in industry to separate a product from impurities or other products. The original mixture may either be a natural resource or the product of a chemical reaction.
An oil water separator (OWS) is a piece of equipment used to separate oil and water mixtures into their separate components. There are many different types of oil-water separator. Each has different oil separation capability and are used in different industries. Oil water separators are designed and selected after consideration of oil separation performance parameters and life cycle cost considerations. "Oil" can be taken to mean mineral, vegetable and animal oils, and the many different hydrocarbons.

Instant tea is a powdered mix in which water is added, in order to reconstitute it into a cup of tea. The earliest form of instant tea was developed in the United Kingdom in 1885. A patent was granted for a paste made of concentrated tea extract, sugar, and evaporated milk, which became tea when hot water was added. However, no notable developments were made until spray drying technology allowed for drying the tea concentrates at a temperature which did not damage the flavors of the product.