A mitochondrion is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term mitochondrion was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name.

5' AMP-activated protein kinase or AMPK or 5' adenosine monophosphate-activated protein kinase is an enzyme that plays a role in cellular energy homeostasis, largely to activate glucose and fatty acid uptake and oxidation when cellular energy is low. It belongs to a highly conserved eukaryotic protein family and its orthologues are SNF1 in yeast, and SnRK1 in plants. It consists of three proteins (subunits) that together make a functional enzyme, conserved from yeast to humans. It is expressed in a number of tissues, including the liver, brain, and skeletal muscle. In response to binding AMP and ADP, the net effect of AMPK activation is stimulation of hepatic fatty acid oxidation, ketogenesis, stimulation of skeletal muscle fatty acid oxidation and glucose uptake, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipogenesis, inhibition of adipocyte lipolysis, and modulation of insulin secretion by pancreatic β-cells.
The ERRs are orphan nuclear receptors, meaning the identity of their endogenous ligand has yet to be unambiguously determined. They are named because of sequence homology with estrogen receptors, but do not appear to bind estrogens or other tested steroid hormones.
The unfolded protein response (UPR) is a cellular stress response related to the endoplasmic reticulum (ER) stress. It has been found to be conserved between mammalian species, as well as yeast and worm organisms.

The tumor suppressor gene FLCN encodes the protein folliculin, also known as Birt–Hogg–Dubé syndrome protein, which functions as an inhibitor of Lactate Dehydrogenase-A and a regulator of the Warburg effect. Folliculin (FLCN) is also associated with Birt–Hogg–Dubé syndrome, which is an autosomal dominant inherited cancer syndrome in which affected individuals are at risk for the development of benign cutaneous tumors (folliculomas), pulmonary cysts, and kidney tumors.
The Randle cycle, also known as the glucose fatty-acid cycle, is a metabolic process involving the competition of glucose and fatty acids for substrates. It is theorized to play a role in explaining type 2 diabetes and insulin resistance.

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis, but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.

Mitofusin-2 is a protein that in humans is encoded by the MFN2 gene. Mitofusins are GTPases embedded in the outer membrane of the mitochondria. In mammals MFN1 and MFN2 are essential for mitochondrial fusion. In addition to the mitofusins, OPA1 regulates inner mitochondrial membrane fusion, and DRP1 is responsible for mitochondrial fission.

Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a protein that in humans is encoded by the PPARGC1A gene. PPARGC1A is also known as human accelerated region 20 (HAR20). It may, therefore, have played a key role in differentiating humans from apes.

Nuclear respiratory factor 1, also known as Nrf1, Nrf-1, NRF1 and NRF-1, encodes a protein that homodimerizes and functions as a transcription factor which activates the expression of some key metabolic genes regulating cellular growth and nuclear genes required for respiration, heme biosynthesis, and mitochondrial DNA transcription and replication. The protein has also been associated with the regulation of neurite outgrowth. Alternate transcriptional splice variants, which encode the same protein, have been characterized. Additional variants encoding different protein isoforms have been described but they have not been fully characterized. Confusion has occurred in bibliographic databases due to the shared symbol of NRF1 for this gene and for "nuclear factor -like 1" which has an official symbol of NFE2L1.

Estrogen-related receptor alpha (ERRα), also known as NR3B1, is a nuclear receptor that in humans is encoded by the ESRRA gene. ERRα was originally cloned by DNA sequence homology to the estrogen receptor alpha, but subsequent ligand binding and reporter-gene transfection experiments demonstrated that estrogens did not regulate ERRα. Currently, ERRα is considered an orphan nuclear receptor.

Fibroblast growth factor 21 is a protein that in mammals is encoded by the FGF21 gene. The protein encoded by this gene is a member of the fibroblast growth factor (FGF) family and specifically a member of the endocrine subfamily which includes FGF23 and FGF15/19. FGF21 is the primary endogenous agonist of the FGF21 receptor, which is composed of the co-receptors FGF receptor 1 and β-Klotho.
Mitophagy is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. The process of mitophagy was first described over a hundred years ago by Margaret Reed Lewis and Warren Harmon Lewis. Ashford and Porter used electron microscopy to observe mitochondrial fragments in liver lysosomes by 1962, and a 1977 report suggested that "mitochondria develop functional alterations which would activate autophagy." The term "mitophagy" was in use by 1998.

Fibronectin type III domain-containing protein 5, the precursor of irisin, is a type I transmembrane glycoprotein that is encoded by the FNDC5 gene. Irisin is a cleaved version of FNDC5, named after the Greek messenger goddess Iris.
Mitochondrial fission is the process where mitochondria divide or segregate into two separate mitochondrial organelles. Mitochondrial fission is counteracted by the process of mitochondrial fusion, whereby two separate mitochondria can fuse together to form a large one. Mitochondrial fusion in turn can result in elongated mitochondrial networks. Both mitochondrial fission and fusion are balanced in the cell, and mutations interfering with either processes are associated with a variety of diseases. Mitochondria can divide by prokaryotic binary fission and since they require mitochondrial DNA for their function, fission is coordinated with DNA replication. Some of the proteins that are involved in mitochondrial fission have been identified and some of them are associated with mitochondrial diseases. Mitochondrial fission has significant implications in stress response and apoptosis.
Shelterin is a protein complex known to protect telomeres in many eukaryotes from DNA repair mechanisms, as well as to regulate telomerase activity. In mammals and other vertebrates, telomeric DNA consists of repeating double-stranded 5'-TTAGGG-3' (G-strand) sequences along with the 3'-AATCCC-5' (C-strand) complement, ending with a 50-400 nucleotide 3' (G-strand) overhang. Much of the final double-stranded portion of the telomere forms a T-loop (Telomere-loop) that is invaded by the 3' (G-strand) overhang to form a small D-loop (Displacement-loop).
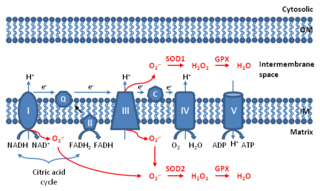
Mitochondrial ROS are reactive oxygen species (ROS) that are produced by mitochondria. Generation of mitochondrial ROS mainly takes place at the electron transport chain located on the inner mitochondrial membrane during the process of oxidative phosphorylation. Leakage of electrons at complex I and complex III from electron transport chains leads to partial reduction of oxygen to form superoxide. Subsequently, superoxide is quickly dismutated to hydrogen peroxide by two dismutases including superoxide dismutase 2 (SOD2) in mitochondrial matrix and superoxide dismutase 1 (SOD1) in mitochondrial intermembrane space. Collectively, both superoxide and hydrogen peroxide generated in this process are considered as mitochondrial ROS.

Mitochondria are dynamic organelles with the ability to fuse and divide (fission), forming constantly changing tubular networks in most eukaryotic cells. These mitochondrial dynamics, first observed over a hundred years ago are important for the health of the cell, and defects in dynamics lead to genetic disorders. Through fusion, mitochondria can overcome the dangerous consequences of genetic malfunction. The process of mitochondrial fusion involves a variety of proteins that assist the cell throughout the series of events that form this process.

Perilipin 5, also known as Oxpatperilipin 5 or PLIN5, is a protein that belongs to perilipin family. This protein group has been shown to be responsible for lipid droplet's biogenesis, structure and degradation. In particular, Perilipin 5 is a lipid droplet-associated protein whose function is to keep the balance between lipolysis and lipogenesis, as well as maintaining lipid droplet homeostasis. For example, in oxidative tissues, muscular tissues and cardiac tissues, PLIN5 promotes association between lipid droplets and mitochondria.

David A. Hood is a Canadian professor, exercise physiologist, and Director of the Muscle Health Research Centre at York University. A holder of an NSERC Tier I Canada Research Chair in Cell Physiology, Hood is credited with making significant research advances in understanding of the biology of exercise, mitochondria and muscle health.