Related Research Articles

Colorectal cancer (CRC), also known as bowel cancer, colon cancer, or rectal cancer, is the development of cancer from the colon or rectum. Signs and symptoms may include blood in the stool, a change in bowel movements, weight loss, and fatigue. Most colorectal cancers are due to old age and lifestyle factors, with only a small number of cases due to underlying genetic disorders. Risk factors include diet, obesity, smoking, and lack of physical activity. Dietary factors that increase the risk include red meat, processed meat, and alcohol. Another risk factor is inflammatory bowel disease, which includes Crohn's disease and ulcerative colitis. Some of the inherited genetic disorders that can cause colorectal cancer include familial adenomatous polyposis and hereditary non-polyposis colon cancer; however, these represent less than 5% of cases. It typically starts as a benign tumor, often in the form of a polyp, which over time becomes cancerous.

Gardner's syndrome is a subtype of familial adenomatous polyposis (FAP). Gardner syndrome is an autosomal dominant form of polyposis characterized by the presence of multiple polyps in the colon together with tumors outside the colon. The extracolonic tumors may include osteomas of the skull, thyroid cancer, epidermoid cysts, fibromas, as well as the occurrence of desmoid tumors in approximately 15% of affected individuals.
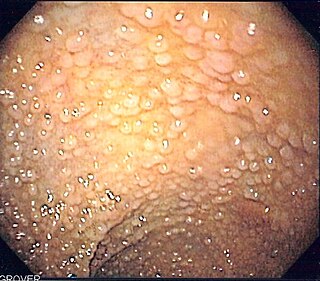
Familial adenomatous polyposis (FAP) is an autosomal dominant inherited condition in which numerous adenomatous polyps form mainly in the epithelium of the large intestine. While these polyps start out benign, malignant transformation into colon cancer occurs when they are left untreated. Three variants are known to exist, FAP and attenuated FAP are caused by APC gene defects on chromosome 5 while autosomal recessive FAP is caused by defects in the MUTYH gene on chromosome 1. Of the three, FAP itself is the most severe and most common; although for all three, the resulting colonic polyps and cancers are initially confined to the colon wall. Detection and removal before metastasis outside the colon can greatly reduce and in many cases eliminate the spread of cancer.

Hereditary nonpolyposis colorectal cancer (HNPCC) or Lynch syndrome is an autosomal dominant genetic condition that is associated with a high risk of colon cancer as well as other cancers including endometrial cancer, ovary, stomach, small intestine, hepatobiliary tract, upper urinary tract, brain, and skin. The increased risk for these cancers is due to inherited genetic mutations that impair DNA mismatch repair. It is a type of cancer syndrome. Because patients with Lynch syndrome can have polyps, the term HNPCC has fallen out of favor.

A neoplasm is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists in growing abnormally, even if the original trigger is removed. This abnormal growth usually forms a mass, when it may be called a tumour or tumor.

Mismatch repair cancer syndrome (MMRCS) is a cancer syndrome associated with biallelic DNA mismatch repair mutations. It is also known as Turcot syndrome and by several other names.
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer whereas the majority of mutations do not.

DNA mismatch repair (MMR) is a system for recognizing and repairing erroneous insertion, deletion, and mis-incorporation of bases that can arise during DNA replication and recombination, as well as repairing some forms of DNA damage.
Adenomatous polyposis coli (APC) also known as deleted in polyposis 2.5 (DP2.5) is a protein that in humans is encoded by the APC gene. The APC protein is a negative regulator that controls beta-catenin concentrations and interacts with E-cadherin, which are involved in cell adhesion. Mutations in the APC gene may result in colorectal cancer and desmoid tumors.

Fundic gland polyposis is a medical syndrome where the fundus and the body of the stomach develop many fundic gland polyps. The condition has been described both in patients with familial adenomatous polyposis (FAP) and attenuated variants (AFAP), and in patients in whom it occurs sporadically.

Microsatellite instability (MSI) is the condition of genetic hypermutability that results from impaired DNA mismatch repair (MMR). The presence of MSI represents phenotypic evidence that MMR is not functioning normally.

Muir–Torre syndrome is a rare hereditary, autosomal dominant cancer syndrome that is thought to be a subtype of HNPCC. Individuals are prone to develop cancers of the colon, genitourinary tract, and skin lesions, such as keratoacanthomas and sebaceous tumors. The genes affected are MLH1, MSH2, and more recently, MSH6, and are involved in DNA mismatch repair.
DNA mismatch repair protein Msh2 also known as MutS homolog 2 or MSH2 is a protein that in humans is encoded by the MSH2 gene, which is located on chromosome 2. MSH2 is a tumor suppressor gene and more specifically a caretaker gene that codes for a DNA mismatch repair (MMR) protein, MSH2, which forms a heterodimer with MSH6 to make the human MutSα mismatch repair complex. It also dimerizes with MSH3 to form the MutSβ DNA repair complex. MSH2 is involved in many different forms of DNA repair, including transcription-coupled repair, homologous recombination, and base excision repair.

DNA mismatch repair protein Mlh1 or MutL protein homolog 1 is a protein that in humans is encoded by the MLH1 gene located on chromosome 3. It is a gene commonly associated with hereditary nonpolyposis colorectal cancer. Orthologs of human MLH1 have also been studied in other organisms including mouse and the budding yeast Saccharomyces cerevisiae.

A colorectal polyp is a polyp occurring on the lining of the colon or rectum. Untreated colorectal polyps can develop into colorectal cancer.

O6-alkylguanine DNA alkyltransferase (also known as AGT, MGMT or AGAT) is a protein that in humans is encoded by the O6-methylguanine DNA methyltransferase (MGMT) gene. O6-methylguanine DNA methyltransferase is crucial for genome stability. It repairs the naturally occurring mutagenic DNA lesion O6-methylguanine back to guanine and prevents mismatch and errors during DNA replication and transcription. Accordingly, loss of MGMT increases the carcinogenic risk in mice after exposure to alkylating agents. The two bacterial isozymes are Ada and Ogt.

Axin-2 also known as axin-like protein (Axil) or axis inhibition protein 2 (AXIN2) or conductin is a protein that in humans is encoded by the AXIN2 gene.
Somatic evolution is the accumulation of mutations and epimutations in somatic cells during a lifetime, and the effects of those mutations and epimutations on the fitness of those cells. This evolutionary process has first been shown by the studies of Bert Vogelstein in colon cancer. Somatic evolution is important in the process of aging as well as the development of some diseases, including cancer.

The histopathology of colorectal cancer of the adenocarcinoma type involves analysis of tissue taken from a biopsy or surgery. A pathology report contains a description of the microscopical characteristics of the tumor tissue, including both tumor cells and how the tumor invades into healthy tissues and finally if the tumor appears to be completely removed. The most common form of colon cancer is adenocarcinoma, constituting between 95% and 98% of all cases of colorectal cancer. Other, rarer types include lymphoma, adenosquamous and squamous cell carcinoma. Some subtypes have been found to be more aggressive.
hPG80 refers to the extracellular and oncogenic version of progastrin. This name first appeared in a scientific publication in January 2020. Until that date, scientific publications only mention 'progastrin', without necessarily explicitly specifying whether it is intracellular or extracellular in the tumor pathological setting.
References
- ↑ Golovko, D; Kedrin, D; Yilmaz, ÖH; Roper, J (2015). "Colorectal cancer models for novel drug discovery". Expert Opinion on Drug Discovery. 10 (11): 1217–29. doi:10.1517/17460441.2015.1079618. PMC 4872297 . PMID 26295972.
- ↑ Oh, BY; Hong, HK; Lee, WY; Cho, YB (28 February 2017). "Animal models of colorectal cancer with liver metastasis". Cancer Letters. 387: 114–120. doi:10.1016/j.canlet.2016.01.048. PMID 26850374.
- ↑ Evans, JP; Sutton, PA; Winiarski, BK; Fenwick, SW; Malik, HZ; Vimalachandran, D; Tweedle, EM; Costello, E; Palmer, DH; Park, BK; Kitteringham, NR (February 2016). "From mice to men: Murine models of colorectal cancer for use in translational research". Critical Reviews in Oncology/Hematology. 98: 94–105. doi:10.1016/j.critrevonc.2015.10.009. PMID 26558688.
- ↑ Groden J, Thliveris A, Samowitz W, et al. (1991). "Identification and characterization of the familial adenomatous polyposis coli gene". Cell . 66 (3): 589–600. doi:10.1016/0092-8674(81)90021-0. PMID 1651174. S2CID 38325198.
- ↑ Moser AR, Pitot HC, Dove WF (1990). "A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse". Science . 247 (4940): 322–4. doi:10.1126/science.2296722. PMID 2296722.
- 1 2 Fodde R, Edelmann W, Yang K, et al. (1994). "A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors". Proc. Natl. Acad. Sci. U.S.A. 91 (19): 8969–73. Bibcode:1994PNAS...91.8969F. doi: 10.1073/pnas.91.19.8969 . PMC 44728 . PMID 8090754.
- ↑ Oshima M, Oshima H, Kitagawa K, Kobayashi M, Itakura C, Taketo M (1995). "Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene". Proc. Natl. Acad. Sci. U.S.A. 92 (10): 4482–6. Bibcode:1995PNAS...92.4482O. doi: 10.1073/pnas.92.10.4482 . PMC 41968 . PMID 7753829.
- ↑ Taketo MM (2006). "Mouse models of gastrointestinal tumors". Cancer Sci. 97 (5): 355–61. doi: 10.1111/j.1349-7006.2006.00190.x . PMID 16630131.
- ↑ Aoki K, Tamai Y, Horiike S, Oshima M, Taketo MM (2003). "Colonic polyposis caused by mTOR-mediated chromosomal instability in Apc+/Delta716 Cdx2+/- compound mutant mice". Nat. Genet. 35 (4): 323–30. doi:10.1038/ng1265. hdl: 2433/147469 . PMID 14625550. S2CID 22623315.
- ↑ Dietrich WF, Lander ES, Smith JS, et al. (1993). "Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse". Cell . 75 (4): 631–9. doi:10.1016/0092-8674(93)90484-8. PMID 8242739.
- ↑ Su LK, Vogelstein B, Kinzler KW (1993). "Association of the APC tumor suppressor protein with catenins". Science . 262 (5140): 1734–7. Bibcode:1993Sci...262.1734S. doi:10.1126/science.8259519. PMID 8259519.
- ↑ Harada N, Tamai Y, Ishikawa T, et al. (1999). "Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene". EMBO J. 18 (21): 5931–42. doi:10.1093/emboj/18.21.5931. PMC 1171659 . PMID 10545105.
- ↑ Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM (1998). "Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes". Cell . 92 (5): 645–56. doi: 10.1016/S0092-8674(00)81132-0 . PMID 9506519.
- ↑ Kolodner R (1996). "Biochemistry and genetics of eukaryotic mismatch repair". Genes Dev. 10 (12): 1433–42. doi: 10.1101/gad.10.12.1433 . PMID 8666228.
- ↑ Edelmann W, Yang K, Umar A, et al. (1997). "Mutation in the mismatch repair gene Msh6 causes cancer susceptibility". Cell . 91 (4): 467–77. doi: 10.1016/S0092-8674(00)80433-X . PMID 9390556.
- ↑ Reitmair AH, Redston M, Cai JC, et al. (1996). "Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice". Cancer Res. 56 (16): 3842–9. PMID 8706033.
- ↑ Baker SM, Plug AW, Prolla TA, et al. (1996). "Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over". Nat. Genet. 13 (3): 336–42. doi:10.1038/ng0796-336. PMID 8673133. S2CID 37096830.
- ↑ Edelmann W, Yang K, Kuraguchi M, et al. (1999). "Tumorigenesis in Mlh1 and Mlh1/Apc1638N mutant mice". Cancer Res. 59 (6): 1301–7. PMID 10096563.
- ↑ Reitmair AH, Cai JC, Bjerknes M, et al. (1996). "MSH2 deficiency contributes to accelerated APC-mediated intestinal tumorigenesis". Cancer Res. 56 (13): 2922–6. PMID 8674041.
- ↑ Baker SM, Harris AC, Tsao JL, et al. (1998). "Enhanced intestinal adenomatous polyp formation in Pms2-/-;Min mice". Cancer Res. 58 (6): 1087–9. PMID 9515784.
- ↑ Engle SJ, Hoying JB, Boivin GP, Ormsby I, Gartside PS, Doetschman T (1999). "Transforming growth factor beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis". Cancer Res. 59 (14): 3379–86. PMID 10416598.
- ↑ Janssen KP, el-Marjou F, Pinto D, et al. (2002). "Targeted expression of oncogenic K- ras in intestinal epithelium causes spontaneous tumorigenesis in mice". Gastroenterology . 123 (2): 492–504. doi: 10.1053/gast.2002.34786 . PMID 12145803.
- ↑ Calcagno SR, Li S, Colon M, et al. (2008). "Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon". Int. J. Cancer . 122 (11): 2462–70. doi:10.1002/ijc.23383. PMC 3908548 . PMID 18271008.
- ↑ Velcich A, Yang W, Heyer J, et al. (2002). "Colorectal cancer in mice genetically deficient in the mucin Muc2". Science . 295 (5560): 1726–9. Bibcode:2002Sci...295.1726V. doi:10.1126/science.1069094. PMID 11872843. S2CID 19425315.
- ↑ Berg DJ, Davidson N, Kühn R, et al. (1996). "Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses". J. Clin. Invest. 98 (4): 1010–20. doi:10.1172/JCI118861. PMC 507517 . PMID 8770874.
- ↑ Shah SA, Simpson SJ, Brown LF, et al. (1998). "Development of colonic adenocarcinomas in a mouse model of ulcerative colitis". Inflamm. Bowel Dis. 4 (3): 196–202. doi: 10.1002/ibd.3780040305 . PMID 9741021. S2CID 39002611.
- ↑ Hermiston ML, Gordon JI (1995). "Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin". Science . 270 (5239): 1203–7. Bibcode:1995Sci...270.1203H. doi:10.1126/science.270.5239.1203. PMID 7502046. S2CID 19696029.
- 1 2 3 Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H (2011). "Carcinogenicity of deoxycholate, a secondary bile acid". Arch. Toxicol. 85 (8): 863–71. doi:10.1007/s00204-011-0648-7. PMC 3149672 . PMID 21267546.
- 1 2 3 Prasad AR, Prasad S, Nguyen H, Facista A, Lewis C, Zaitlin B, Bernstein H, Bernstein C (2014). "Novel diet-related mouse model of colon cancer parallels human colon cancer". World J Gastrointest Oncol. 6 (7): 225–43. doi:10.4251/wjgo.v6.i7.225. PMC 4092339 . PMID 25024814.
- ↑ Neufert C, Becker C, Neurath MF (2007). "An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression". Nat Protoc . 2 (8): 1998–2004. doi:10.1038/nprot.2007.279. PMID 17703211. S2CID 28778351.
- ↑ Poole AJ, Heap D, Carroll RE, Tyner AL (2004). "Tumor suppressor functions for the Cdk inhibitor p21 in the mouse colon". Oncogene . 23 (49): 8128–34. doi: 10.1038/sj.onc.1207994 . PMID 15377995.
- ↑ Maltzman T, Whittington J, Driggers L, Stephens J, Ahnen D (1997). "AOM-induced mouse colon tumors do not express full-length APC protein". Carcinogenesis . 18 (12): 2435–9. doi: 10.1093/carcin/18.12.2435 . PMID 9450492.
- ↑ Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H (2003). "A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate". Cancer Sci. 94 (11): 965–73. doi:10.1111/j.1349-7006.2003.tb01386.x. PMID 14611673. S2CID 6538164.