
In physics, chemistry, and thermodynamics, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars.
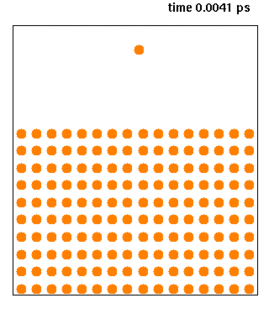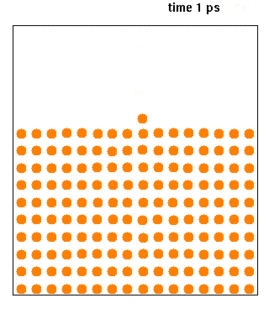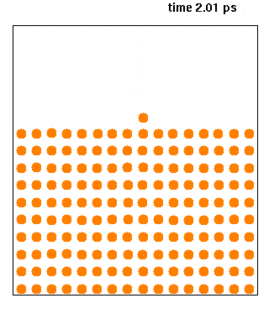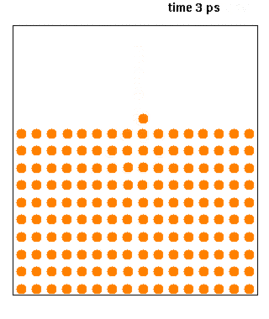
Molecular dynamics (MD) is a computer simulation method for analyzing the physical movements of atoms and molecules. The atoms and molecules are allowed to interact for a fixed period of time, giving a view of the dynamic "evolution" of the system. In the most common version, the trajectories of atoms and molecules are determined by numerically solving Newton's equations of motion for a system of interacting particles, where forces between the particles and their potential energies are often calculated using interatomic potentials or molecular mechanics force fields. The method is applied mostly in chemical physics, materials science, and biophysics.

The Lennard-Jones potential is an intermolecular pair potential. Out of all the intermolecular potentials, the Lennard-Jones potential is the one that has been the most extensively studied. It is considered an archetype model for simple yet realistic intermolecular interactions.

Molecular modelling encompasses all methods, theoretical and computational, used to model or mimic the behaviour of molecules. The methods are used in the fields of computational chemistry, drug design, computational biology and materials science to study molecular systems ranging from small chemical systems to large biological molecules and material assemblies. The simplest calculations can be performed by hand, but inevitably computers are required to perform molecular modelling of any reasonably sized system. The common feature of molecular modelling methods is the atomistic level description of the molecular systems. This may include treating atoms as the smallest individual unit, or explicitly modelling protons and neutrons with its quarks, anti-quarks and gluons and electrons with its photons.

PLATO is a suite of programs for electronic structure calculations. It receives its name from the choice of basis set used to expand the electronic wavefunctions.
This page provides supplementary chemical data on formic acid.
ABINIT is an open-source suite of programs for materials science, distributed under the GNU General Public License. ABINIT implements density functional theory, using a plane wave basis set and pseudopotentials, to compute the electronic density and derived properties of materials ranging from molecules to surfaces to solids. It is developed collaboratively by researchers throughout the world. A web-based easy-to-use graphical version, which includes access to a limited set of ABINIT's full functionality, is available for free use through the nanohub.

In the context of chemistry and molecular modelling, a force field is a computational method that is used to estimate the forces between atoms within molecules and also between molecules. More precisely, the force field refers to the functional form and parameter sets used to calculate the potential energy of a system of atoms or coarse-grained particles in molecular mechanics, molecular dynamics, or Monte Carlo simulations. The parameters for a chosen energy function may be derived from experiments in physics and chemistry, calculations in quantum mechanics, or both. Force fields are interatomic potentials and utilize the same concept as force fields in classical physics, with the difference that the force field parameters in chemistry describe the energy landscape, from which the acting forces on every particle are derived as a gradient of the potential energy with respect to the particle coordinates.

Professor Ian Philip Grant, DPhil; FRS; CMath; FIMA, FRAS, FInstP is a British mathematical physicist. He is Emeritus Professor of Mathematical Physics at the University of Oxford and was elected a fellow of the Royal Society in 1992. He is a pioneer in the field of computational physics and is internationally recognised as the principal author of GRASP, the General Relativistic Atomic Structure Program.
A field-theoretic simulation is a numerical strategy to calculate structure and physical properties of a many-particle system within the framework of a statistical field theory, like e.g. a polymer field theory. A convenient possibility is to use Monte Carlo (MC) algorithms, to sample the full partition function integral expressed in field-theoretic representation. The procedure is then called the auxiliary field Monte Carlo method. However, it is well known that MC sampling in conjunction with the basic field-theoretic representation of the partition function integral, directly obtained via the Hubbard-Stratonovich transformation, is impracticable, due to the so-called numerical sign problem. The difficulty is related to the complex and oscillatory nature of the resulting distribution function, which causes a bad statistical convergence of the ensemble averages of the desired structural and thermodynamic quantities. In such cases special analytical and numerical techniques are required to accelerate the statistical convergence of the field-theoretic simulation.

Molecular Dynamics of Mixtures (MDynaMix) is a computer software package for general purpose molecular dynamics to simulate mixtures of molecules, interacting by AMBER- and CHARMM-like force fields in periodic boundary conditions. Algorithms are included for NVE, NVT, NPT, anisotropic NPT ensembles, and Ewald summation to treat electrostatic interactions. The code was written in a mix of Fortran 77 and 90. The package runs on Unix and Unix-like (Linux) workstations, clusters of workstations, and on Windows in sequential mode.
Particle–Particle–Particle–Mesh (P3M) is a Fourier-based Ewald summation method to calculate potentials in N-body simulations.
This is a list of notable computer programs that are used for nucleic acids simulations.

Ascalaph Designer is a computer program for general purpose molecular modelling for molecular design and simulations. It provides a graphical environment for the common programs of quantum and classical molecular modelling ORCA, NWChem, Firefly, CP2K and MDynaMix . The molecular mechanics calculations cover model building, energy optimizations and molecular dynamics. Firefly covers a wide range of quantum chemistry methods. Ascalaph Designer is free and open-source software, released under the GNU General Public License, version 2 (GPLv2).

Interatomic potentials are mathematical functions to calculate the potential energy of a system of atoms with given positions in space. Interatomic potentials are widely used as the physical basis of molecular mechanics and molecular dynamics simulations in computational chemistry, computational physics and computational materials science to explain and predict materials properties. Examples of quantitative properties and qualitative phenomena that are explored with interatomic potentials include lattice parameters, surface energies, interfacial energies, adsorption, cohesion, thermal expansion, and elastic and plastic material behavior, as well as chemical reactions.
PLUMED is an open-source library implementing enhanced-sampling algorithms, various free-energy methods, and analysis tools for molecular dynamics simulations. It is designed to be used together with ACEMD, AMBER, DL_POLY, GROMACS, LAMMPS, NAMD, OpenMM, ABIN, CP2K, i-PI, PINY-MD, and Quantum ESPRESSO, but it can also be used to together with analysis and visualization tools VMD, HTMD, and OpenPathSampling.
The Mie potential is an intermolecular pair potential, i.e. it describes the interactions between particles at the atomic level.
Statistical associating fluid theory (SAFT) is a chemical theory, based on perturbation theory, that uses statistical thermodynamics to explain how complex fluids and fluid mixtures form associations through hydrogen bonds. Widely used in industry and academia, it has become a standard approach for describing complex mixtures. Since it was first proposed in 1990, SAFT has been used in a large number of molecular-based equation of state models for describing the Helmholtz energy contribution due to association.










