
Gobiidae or gobies is a family of bony fish in the order Gobiiformes, one of the largest fish families comprising more than 2,000 species in more than 200 genera. Most of gobiid fish are relatively small, typically less than 10 cm (3.9 in) in length, and the family includes some of the smallest vertebrates in the world, such as Trimmatom nanus and Pandaka pygmaea, Trimmatom nanus are under 1 cm long when fully grown, then Pandaka pygmaea standard length are 9 mm (0.35 in), maximum known standard length are 11 mm (0.43 in). Some large gobies can reach over 30 cm (0.98 ft) in length, but that is exceptional. Generally, they are benthic or bottom-dwellers. Although few are important as food fish for humans, they are of great significance as prey species for other commercially important fish such as cod, haddock, sea bass and flatfish. Several gobiids are also of interest as aquarium fish, such as the dartfish of the genus Ptereleotris. Phylogenetic relationships of gobiids have been studied using molecular data.

Fundulus is a genus of ray-finned fishes in the superfamily Funduloidea, family Fundulidae. It belongs to the order of toothcarps (Cyprinodontiformes), and therein the large suborder Cyprinodontoidei. Most of its closest living relatives are egg-laying, with the notable exception of the splitfin livebearers (Goodeidae).
Euryhaline organisms are able to adapt to a wide range of salinities. An example of a euryhaline fish is the short-finned molly, Poecilia sphenops, which can live in fresh water, brackish water, or salt water.

The California killifish is a type of killifish (Fundulidae) found along the coast of southern California and Baja California.

Fathead minnow, also known as fathead or tuffy, is a species of temperate freshwater fish belonging to the genus Pimephales of the cyprinid family. The natural geographic range extends throughout much of North America, from central Canada south along the Rockies to Texas, and east to Virginia and the Northeastern United States. This minnow has also been introduced to many other areas via bait bucket releases. Its golden, or xanthic, strain, known as the rosy-red minnow, is a very common feeder fish sold in the United States and Canada. This fish is best known for producing Schreckstoff.

The sheepshead minnow, also known as sheepshead pupfish, is a species of ray-finned fish in the family Cyprinodontidae, the pupfishes. It is found in salt marsh and estuary environments and is native to the eastern coasts of North and Central America.

Lepidophagy is a specialised feeding behaviour in fish that involves eating the scales of other fish. Lepidophagy is widespread, having evolved independently in at least five freshwater families and seven marine families. A related feeding behavior among fish is pterygophagy: feeding on the fins of other fish.
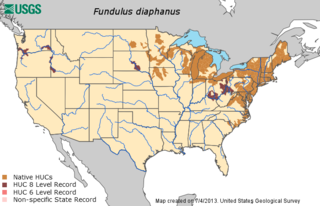
The banded killifish is a North American species of temperate freshwater killifish belonging to the genus Fundulus of the family Fundulidae. Its natural geographic range extends from Newfoundland to South Carolina, and west to Minnesota, including the Great Lakes drainages. This species is the only freshwater killifish found in the northeastern United States. While it is primarily a freshwater species, it can occasionally be found in brackish water.

The mangrove rivulus or mangrove killifish, Kryptolebias marmoratus, is a species of killifish in the family Rivulidae. It lives in brackish and marine waters along the coasts of Florida, through the Antilles, and along the eastern and northern Atlantic coasts of Mexico, Central America and South America. It has a very wide tolerance of both salinity and temperature, can survive for about two months on land, and mostly breeds by self-fertilization. It is typically found in areas with red mangrove and sometimes lives in burrows of Cardisoma guanhumi crabs.

Judith Shulman Weis is an American marine biologist. Her research and writing focuses on estuarine ecology and ecotoxicology, including the responses of salt marsh and brackish marsh organisms, populations and communities to stresses, particularly heavy metal contaminants, invasive species and parasites. She is also working to reduce the spread of microplastics in the environment and find solutions to protecting coastal marshes from sea level rise.

The rainwater killifish is a small silvery fish with yellow flashes and diamond shaped scales that is widespread from Cape Cod, Massachusetts, through to Tampico, Mexico. It is commonly found in large numbers in fresh to brackish estuarine environments. It feeds on tiny crustaceans, mosquito larvae, small worms, and mollusks. It can reach up to 62 mm.

The desert pupfish is a rare species of bony fish in the family Cyprinodontidae. It is a small fish, typically less than 7.62 cm (3 in) in length. Males are generally larger than females, and have bright-blue coloration, while females and juveniles are silvery or tan. A notable attribute of the desert pupfish is their ability to survive in environments of extreme salinity, pH, and temperature, and low oxygen content. The desert pupfish mates in a characteristic fashion, wherein compatible males and females will come in contact and collectively jerk in an s-shape. Each jerk typically produces a single egg that is fertilized by the male and deposited in his territory. Breeding behavior includes aggressive arena-breeding and more docile consort-pair breeding.

The southern studfish is a ray-finned fish of the family Fundulidae, the tooth carps, that is native to the southeastern United States.
Fish are exposed to large oxygen fluctuations in their aquatic environment since the inherent properties of water can result in marked spatial and temporal differences in the concentration of oxygen. Fish respond to hypoxia with varied behavioral, physiological, and cellular responses to maintain homeostasis and organism function in an oxygen-depleted environment. The biggest challenge fish face when exposed to low oxygen conditions is maintaining metabolic energy balance, as 95% of the oxygen consumed by fish is used for ATP production releasing the chemical energy of nutrients through the mitochondrial electron transport chain. Therefore, hypoxia survival requires a coordinated response to secure more oxygen from the depleted environment and counteract the metabolic consequences of decreased ATP production at the mitochondria.
Homalometron pallidum is a species of marine trematodes in the family Apocreadiidae. It is an endoparasite of the mummichog, Fundulus heteroclitus, a small fish found in brackish water along the east coast of the United States and Canada. It has a complex life cycle and lives inside several different host species at different stages.

The plains topminnow is a species of freshwater topminnow found in North America. The fish has a small range within the United States of America which consists of two major populations.

The Gulf killifish is one of the largest members of the genus Fundulus; it is capable of growing up to 7 inches (18 cm) in length, whereas the majority of other Fundulus reach a maximum length of 4 inches (10 cm). Therefore, F. grandis is among the largest minnows preyed upon by many sport fish, such as flounder, speckled trout, and red drum. Fundulus derives from the Latin meaning "bottom," and grandis means "large". The Gulf killifish is native to the Gulf of Mexico from Texas to Florida and the eastern coast of Florida and the Caribbean Sea in the Atlantic Ocean. Threats to the survival of the Gulf killifish include extreme changes in salinity, changes in temperatures, and toxic events such as the hypoxic dead zone in Louisiana and the Deepwater Horizon oil spill. The Gulf killifish is currently being used to test the effects of oil and oil dispersants on the physiology of marine species affected by these substances. This is significant to conservation biology, because with the continued extraction of oil and other natural resources from North American waters, it has become increasingly important to understand the risks and consequences in worst-case scenarios, such as the Deepwater Horizon oil spill, and the lasting effects on the marine ecosystem.

The bayou killifish or bayou topminnow is a topminnow-like fish that thrives primarily in the shallow waters off the shores of the Americas, as well as fresh and brackish waters. Feeding off of small vertebrates and invertebrates, this fish displays reproduction techniques unique to its species.

Fundulus luciae, the spotfin killifish, is a member of the genus Fundulus. This hardy fish is notable for spending its entire life in sporadically flooded salt marsh habitat, sheltering in shallow pools, puddles, and small tidal rivulets. It closely resembles the mummichog in shape and coloration, but the two species can be distinguished by dorsal fin ray count: 8–9 in the spotfin versus 11–12 in the mummichog. Additionally, the dorsal fin of F. luciae originates farther back, and slightly behind the anal fin origin; in the mummichog, the dorsal fin begins anteriorly to the anal fin origin. The spotfin killifish is named for the pronounced ocellus found on the posterior dorsal fin of adult males. It is a small fish, seldom attaining 50 millimetres (2.0 in) in total length. Its distribution extends along the U.S. east coast from Massachusetts to Georgia.
Patricia M. Schulte is a Canadian zoologist who is a Professor of Zoology at the University of British Columbia. Her research considers physiology, genomics and population genetics. Schulte is a Fellow of the Royal Society of Canada and the former President of the Canadian Society of Zoologists.


















